The Polarity of Molecules
We have seen that atoms differ in their capacity to "hold onto" their
electrons; some gain electrons, some lose electrons. Certain atoms,
oxygen and nitrogen, for example, do not have sufficient
electron-attractive power to become fully charged negative ions.
However, the attractions of electrons is sufficiently great so that, when covalently bonded to hydrogen, the electrons are not equally shared between the two nuclei. The electrons tend to spend more time around the oxygen nucleus and consequently less time around the hydrogen nucleus. This means that one portion of a molecule is slightly positive or slightly negative in relation to another portion of the same molecule. When such an uneven distribution of charge occurs, the molecule is said to exhibit polarity. The molecule has a positive and a negative end, separated from eachother like the poles of a bar magnet. Because this is not a full -1 or +1 charge but a smaller charge, it is represented as delta positive or delta negative.
Water is a good example to illustrate this point. Although, as a whole, the water molecule is electrically neutral, it does have a positive and a negative end. The geometric configuration of the molecule places both hydrogen atoms at one end. The nucleus of the oxygen atom attracts electrons more than the nuclei of the hydrogen atoms. This results in two slightly positively charged regions on one end of the molecule and a single slightly negatively charged region on the other. The molecule thus has a positive and a negative end, or two poles. The water molecule is a polar molecule.
The significance of molecular polarity to the biological sciences come
from two main areas: First, polar molecules tend to become oriented with
respect to other molecules. Because of this, polar molecules are
important in helping to establish the three-dimensional structure or
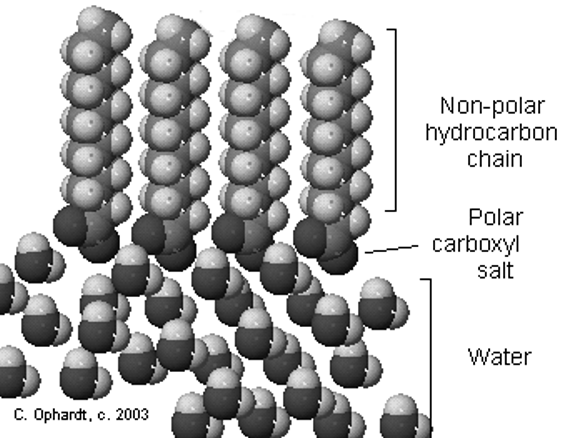
orientation of other larger molecules. For example, molecules of fatty
acids (Chapter 8), found in all living matter are composed of a nonpolar
carbon chain with a polar carbon-oxygen group (COOH) at one end. When
placed in water, the polar ends of the fatty acid molecules are attracted
to water molecules, which are also polar. The nonpolar carbon chains are
at the same time repelled by the water. As a result, fatty acid molecules
are oriented on the water's surface. 
Of particular importance to living things is the orientation of phospholipid molecules, which are a combination of a fat molecule with a phosphate group. Phospholipids are among the most important parts of cell membranes. They tend to become oriented on surface or boundary regions in a manner similar to the fatty acids on water. It is partly in this way that cell membranes are given a distinct structure.
Second, polarity is important in understanding both the geometry and the chemical characteristics of large molecules, such as proteins. Proteins are so large that they may possess a number of polar groups on one molecule. Polar groups, like radicals (Section 3-4), are simply groups of atoms which bear as a unit a partial positive or a partial negative charge. The specific geometry of proteins exists in part because polar groups on one part of the molecule attract polar groups on another part of the same molecule. This stabilizes the specific twisting and folding of the molecule which is all-important to the chemical characteristics it displays.
Polarity thus tends to bring small molecules, or specific regions of large molecules, into definite geometric relation. In this way, the chemical bonding between individual molecules or between specific portions of large molecules is brought ab. Hydrogen bonds are produced by the electrostatic attraction between positively (partially)out more easily.
In living organisms, one of the most common types of chemical bonds produced by polar attraction is the hydrogen bond charged hydrogen atoms (protons) on one part of the molecule, and negatively charged atoms of oxygen or nitrogen on the same or another molecule. The oxygen and nitrogen atoms are partially negatively charged because their nuclei attract large numbers of electrons around them. Because the hydrogen bond occurs between polar regions of a molecule, it is, like all polar attractions, relatively weak.
A simple example of hydrogen bonding can be seen between water molecules. The hydrogen atoms of one water molecule form a hydrogen bond with the oxygen atom of the adjacent molecule. Polar molecules, such as fatty acids, tend to orient themselves in respect to other polar molecules. Here the molecules of a fatty acid line up in a specific fashion on the surface of water. The COOH groups are in the water (also polar) and the carbon chains stick out into the air.