C2005/F2401 '10 Lecture 14
© Copyright 2010 Deborah Mowshowitz
and Lawrence Chasin Department of Biological Sciences Columbia University New York, NY.
Last Edited: 10/26/10 10:02 AM.
Note: The figs & tables in the 6th & 7th ed. of Becker are the same. In the 5th ed., the chapter on translation is chap. 20, not
22, but the fig. numbers are about the same. So fig. 22-x in the 6th or 7th ed. is 20-x in the 5th.
Handouts 14A -- Wobble & Ribosome Structure; 14B -- tRNA Loading
For a video of the class demonstration of translation, go to http://www.columbia.edu/cu/biology/courses/c2005/lectures/translation.wmv
I. Role of Ribosomes in Translation
A. How do ribosomes fit in?
1. Function.
a. You need something to hold tRNA (two loaded ones at a time) onto mRNA while amino acids are being hooked up. (How many weak bonds hold a tRNA and mRNA together?)
b. You need to provide necessary enzymes for making peptide bond etc.
2. Ribosome contains both RNA and protein. Holding of tRNA etc. is done by a structure that contains both RNA(s) and protein(s). Anything made of both is called an RNP = ribonucleoprotein or ribonucleoprotein particle. This particular RNP structure = ribosome; RNA inside it is called ribosomal RNA or rRNA. Be careful not to confuse ribosomal RNA (rRNA) & ribosomes.
For pictures of ribosome structure see Sadava fig. 14.14 (12.10) and/or Becker figs. 22-1 & 22-2 & table 22-1.) Molecular details of structure below.
3. Important Structural Features (See Becker, fig. 22-2 or Sadava fig. 14.14 (12.10) See handout 13-A.
a. 1 site or groove for mRNA.
b. 2 sites for loaded tRNA (hybridized to mRNA) per ribosome -- These are called A and P; more details below. These sites bind both mRNA and (loaded) tRNA.
c. One site for unloaded tRNA This site binds binds empty, used tRNA before it is bumped off the ribosome. (It's called E for exit site). This site is sometimes omitted in diagrams of elongation. (The T site shown in the 7th ed. of Purves probably does not exist and should be ignored.) The E site binds tRNA but not mRNA.
d. All ribosomes are the same. Which protein is made does not depend on the ribosome.
e. Peptidyl transferase is part of the ribosome. (Details below.)
B. How do Ribosomes Work?
1. How Ribosomes Move (See Becker fig. 22-7 & 22-10 or Sadava fig. 14.16 (12.12).
a. Directions: Ribosome moves down mRNA 5' to 3' (or mRNA slides through ribosome) as peptide is made amino to carboxyl. Both peptides and nucleic acids are both made/read as written, left to right.
How mRNA is made and how it is translated happen to be in the same direction, but transcription and translation are two separate processes (which are usually coupled in prokaryotes but not eukaryotes).b. A & P sites. The two binding sites for loaded tRNA are different -- 1 called A binds amino acyl tRNA & 1 called P binds peptidyl tRNA.
c. Translocation -- Movement of mRNA (& tRNA's) relative to the Ribosome.
(1). Differences between the A & P sites allow unidirectional movement. Before peptide bond is formed, AA-tRNA is in A site and peptidyl-tRNA is in P site. As soon as peptide bond is formed, tRNA in A site becomes a peptidyl-tRNA, and tRNA in P site becomes unloaded or empty tRNA, Since "wrong" types of tRNA are now in A & P sites, ribosome no longer fits properly and moves over one codon, shifting peptidyl-tRNA to P site, empty tRNA to E site and leaving A site empty to hold next AA-tRNA. When the next AA-tRNA arrives, the empty or unloaded tRNA is then released to be reloaded and used again.
(2). Which part actually moves? Ribosome or mRNA?
mRNA & ribosome: Move one codon relative to each other. On handout 13B, in steps 5 & 6, it looks like the ribosome moves one codon toward the 3' end of the message. Probably, the ribosome stays in fixed position and the mRNA advances one codon through the ribosome in the 5' direction, as shown in step 2 → 3. (In other words, if drawn correctly, the mRNA moves to left instead of the ribosome moving to the right.)
Messenger RNA & tRNA: These do not move relative to each other but are pulled together.
Note that the effect is the same whether the ribosome or the mRNA (& attached tRNAs) move -- the ribosome and mRNA are shifted one codon relative to each other and all the tRNA's shift down one site. Either way you look at it, the overall result is:
(3). Protein Synthesis uses up a lot of Energy. Movement and binding tRNA both require energy which we are ignoring. You probably need at least 5 P's split from ATP (or GTP) per AA added if you count all the steps involved, not just growth of peptide chain. So making proteins is a very expensive procedure, and making unnecessary proteins is very wasteful. As a result, there has been strong selection for efficient regulation of protein synthesis; how regulation works in bacteria will be explained next time. (For involvement of GTP in translation see Becker figs. 22-8 & 22-10.)
To review how the A & P sites fit in, try problem 7-12, part C.
2. How Ribosomes attach to mRNA
a. Attachment. When not in use, ribosomes come apart into subunits. The cell contains a pool of subunits. When translation starts, one small subunit and one large subunit clamp onto the mRNA to form a ribosome and begin translation. When translation ends, the two subunits come apart, fall off the mRNA, and return to the pool -- ready to be used again.
b. Polysomes -- More than one ribosome can read a single message at one time.
The first ribosome attaches near the 5' end of the mRNA. Then the ribosome moves (see note below) down the mRNA toward the 3' end, making protein. Once the ribosome has moved far enough down, a second ribosome can attach behind it (on the 5' side) and follow the first ribosome down the message. As each ribosome moves toward the 3' end, making protein, another ribosome attaches after it until the entire mRNA is covered with ribosomes. The mRNA remains covered with ribosomes; although some ribosomes finish and fall off the 3' end, others continually attach at the 5' end. The mRNA covered with multiple ribosomes is called a polyribosome or polysome for short. Sadava fig.14.18 (12.14).Note: This description assumes that the ribosomes move down the mRNA, 5' to 3'. The result is the same if you assume the ribosomes stay put while the mRNA moves through the ribosomes, 5' end first. (Which is more likely.) Once enough mRNA has slid through the first ribosome, a second ribosome can attach to the space on the 5' end and the mRNA can thread through that one next, and so on.
To review polysomes, try
problem 7-16, part B.
C. Detailed Structure & Assembly of Ribosomes: (see bottom of handout 14A). See texts for pictures.
1. Parts. Each ribosome is made of two subunits. Each subunit is a ribonucleoprotein or RNP made of at least one kind of rRNA and many proteins. Each subunit is made separately; two subunits (one large, one small) clamp onto message to form a complete ribosome for translation. The two subunits separate from each other (and the mRNA) when they reach the end of the mRNA. See Sadava fig. 14.14 (12.10); Becker fig. 22-1.
2. Names of Parts. Subunits of ribosome and different ribosomal RNA's are identified by their sedimentation constants (S values) in an ultracentrifuge. Two values are given on the handout for the sizes of the RNA's and subunits -- the smaller number is for prokaryotes; the larger # for eukaryotes. See handout and/or Becker table 22-1.
3. Self assembly -- How does ribosome structure form? The structure of each subunit is determined by the primary sequences of the rRNA's and proteins in it. Just as a protein folds up into the most stable (lowest energy) 3D conformation, so rRNA + proteins of each subunit fold into a ribonucleoprotein particle or RNP with proper 3D shape and function.
4. rRNA vs ribosomes. Be careful not to confuse ribosomes with ribosomal RNA.
D. Peptidyl Transferase is a Ribozyme
Peptidyl transferase is part of the
ribosome.
The catalytic activity is a property of the rRNA in the large subunit, not a protein, so
this is not really an enzyme (catalyst made of protein) but a ribozyme (catalyst made of RNA). It
is presumed that it is a relic of the "RNA world" that existed before DNA and protein
took over many of the early functions of RNA (which has both catalytic and informational
properties). Peptidyl transferase is not the only ribozyme -- other catalytic
RNA's are known.
For more details see http://www.sciencemag.org/cgi/content/full/289/5481/878 You can reach this site from any Columbia computer; I don't know if you can
get it from a personal computer if you are not a subscriber to Science Online.
Note that this site has detailed "hypernotes" which list many sites
useful to molecular biologists. If you find any of these useful, please tell Dr.
M. so she can tell other students. (The site maybe slow to load, but the
link works.)
II. Starts & Stops. How do peptide chains get started? How does peptide chain growth stop?
A. Starts -- AUG is used for both 'start' and 'methionine'
1. How
does protein synthesis start? -- you need a special met-tRNA for the
P site.
If the P
site holds only tRNA's with chains, how will the first AA-tRNA fit on the ribosome? The
answer is that there is a special tRNA (initiator tRNA or tRNAmeti) which is used only in starting
chains. This tRNA carries met and recognizes the codon AUG, which is both the (only) codon
for met and the (only) start codon. This AA-tRNA is special in that it only fits in the P
site.
2. How does met get inserted in the middle of chains?-- you need a different met-tRNA for the A site.
If the tRNA for met fits in the P site, how will met be added to a growing chain? There is a second "ordinary" tRNA for met, one that fits in the A site. Both tRNA's for met recognize the same codon and carry the same amino acid, but one fits only in the P site and one only in the A site. The first (initiator tRNA) is used only to start chains and the other is used only in the middle of chains. Sadava fig. 14.15 (12.11) or Becker fig. 22-8 if you are curious about all the details of initiation.
3. Processing. Why don't all protein chains start with methionine? Met is usually removed from the amino end of the protein before the peptide chain folds up. This method of synthesis (having all proteins start with met) is chosen for ease of manufacture, not because met is needed for protein function. It makes synthesis easier but produces a product that needs alterations before use (removal of met). Post synthetic modifications such as removal of met are common (for all macromolecules, not just proteins) and are often called processing. It is not at all unusual for a modification or processing enzyme to take off a few amino acids or nucleotides here or there, add a group or cofactor, etc. These modifications are much more extensive in eukaryotes than in prokaryotes and will be discussed at length in later lectures and/or next term.
4. Finding the right AUG -- How do ribosomes select the right AUG to start at? The process is different in eukaryotes and prokaryotes, and we are leaving the details for more advanced courses. See texts if you are interested.
B. Stops -- No tRNA is involved
1. No stop tRNA. There are no tRNA's for stop codons, so a ribosome stalls when it comes to a stop codon. A tRNA with a peptide chain is now sitting in the P site, but there is no tRNA to fit in the A site.
2. Role of protein release factor. A protein called a release factor binds to the stop codon in the A site and triggers release of the completed peptide chain. The peptide chain is released from its tRNA, folds up, and goes off to do its job.
3. Fate of last tRNA & ribosome. Once the peptide is released from its tRNA, the tRNA falls off the ribosome and then the ribosome disassociates into subunits and the subunits fall off the mRNA. The tRNA & ribosomal subunits can be used again. Sadava fig. 14.17 (12.13) or Becker fig. 22-11.
To review starts and stops, try problems 7-12 G, 7-17, & 7-20 B.
III. Role of Transfer RNA in Translation, cont. -- Wobble & Loading
A. Wobble and Degeneracy -- How many different tRNA's do you need to read all 61 codons for amino acids?
1. Degeneracy vs ambiguity
The genetic code is not ambiguous -- it is always clear what a particular word (codon) means, but there are synonyms -- multiple words (codons) for the same thing (AA). For example. UUU/C (meaning UUU or UUC) is phe. XYU/C always codes for the same AA; XYA/G usually does. (X, Y = any of the 4 bases.) See text or handout with code table. Any code with this property -- multiple words for same thing -- is called degenerate. So the genetic code is degenerate.
2. Wobble
a. Idea: Since the code is degenerate, you could save on the number of different tRNA's required if the same tRNA could be used for two or more synonymous codons. The same tRNA could be used for two or more such codons if you allow a little flexibility in pairing between the base at position 3 of the codon and its corresponding base (position 1) in the anticodon. This flexibility in pairing is known as "wobble." Because of wobble, position 3 of the codon and position 1 of the anticodon are known as "the wobble position" in their respective codons/anticodons.
Note: Wobble = Several different codons read by one tRNA not several different tRNA's used for the same codon. (There may be multiple tRNA's for the same codon, but that's called something else.)
b. Mechanism: By wobble, we mean that the two bases involved in pairing (the ones in the wobble position of the codon and anticodon respectively) can twist slightly relative to each other. Because of this flexibility of alignment, H bonds are possible between groups that don't normally base pair in totally double stranded nucleic acids. {Q&A}. Some examples are drawn in Becker fig. 22-4 and on handout 14 A.
c. Wobble rules: The usual matches allowed at the wobble position are as follows:
| Type of RNA | Position of Base | Base | ||||
| tRNA | 1st position of anticodon | A | C | U | G | I ** |
| mRNA | 3rd position of codon | U | G | A or G | C or U | U, C or A |
** I = inosine (name of nucleoside) or
hypoxanthine (name of base) = A without its amino (has C=O instead of C-NH2
at position 6.)
How I pairs with U, C or A and how U pairs with G is shown on handout 14 A (or
see Becker, fig. 22-4).
d. Example: The same tRNA (with anticodon 3' AAG 5') can pair with both the UUU codon and the UUC codon in mRNA. (Remember pairing is antiparallel, so anticodon must be "upside down" relative to mRNA. 3'AAG 5' in tRNA pairs with 5'UUU or 5' UUC in mRNA.) Since UUU and UUC code for the same amino acid (phe), both can pair with same tRNA carrying phe, and the right amino acid (phe) gets put in every time. So only one tRNA is needed, instead of two, to read both UUU and UUC. (See handout 14 A or fig. 22-4 of Becker.)
Important Note: Not all of the combinations allowed by the "wobble rules" are compatible with the genetic code.
e. How many different tRNA's? On the average, you need two tRNA's for each "box" in the genetic code table. Note that one tRNA can never read more than 3 codons; most tRNA's read two, and some tRNA's read only one codon. So you need about 30 different tRNA's, not close to 64.
What's the exact number needed to read all the codons? Let's start with a possible max. number of 64 and consider the effects of starts, stops, wobble etc. {Q&A}
To review Wobble and Degeneracy, see Problems 7-15, 7-19, & 7-25.
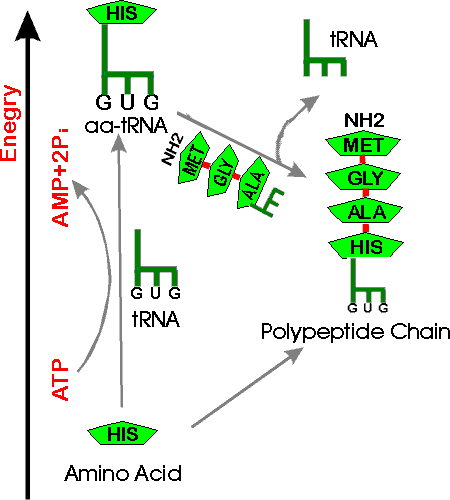
B. Loading of tRNA -- Where does the energy come from to load up tRNA?
1. Overall ATP Balance -- How does ATP fit in? There are two ways to explain how ATP fits in here:
Approach #1 -- uphill/downhill picture. See handout 14 B. (Compare to similar picture for making DNA.)
Basic idea: it's uphill from AA → chain, but downhill from AA-tRNA → chain. 2 P are split off ATP to get the AA onto the tRNA, and it's a downhill slide from there (AA-tRNA) to make the chain. Note that a tRNA-AA connection is broken to add an amino acid, but the connection that is broken is between the last amino acid in the peptide chain and the tRNA, not between the new AA and its tRNA. (The peptide chain adds to the AA on the next tRNA, not the reverse.)

Approach #2 -- sum up the reactions for charging
(rxn 1) tRNA + AA → AA~tRNA (+ water )
(rxn 2) ATP (+ water) → AMP + PPi
(rxn 3) Pyrophosphatase: PPi (+ water) → 2 Pi
Rxn1 is uphill, so you must couple rxn (1) to hydrolysis of ATP (rxn 2).
Δ Go for (1) + (2) is about zero, but PP 'tase = pyrophosphatase removes product (rxn. 3) and pulls the overall reaction to the right (as in nucleic acid synth.). In other words, sum of Δ Go for (1) + (2) + (3) is negative. (Net reaction is shown on the bottom of handout 14B.)
Now the AA~tRNA can be used in protein synthesis to provide free energy for formation of peptide bonds.
Note that there are two different ways to get the energy from two "high energy" bonds from ATP.
Method 1: You split two molecules of 2ATP,
removing one phosphate each time:
2ATP + water → 2ADP + 2Pi
Method 2: You split one molecule of
ATP, removing two phosphates:
ATP + water → AMP + 2Pi.
Either way the "energy cost" is the same. Synthesis of XTP's for nucleic acid synthesis uses the first method; synthesis of AA~tRNA for protein synthesis uses the second method.
2. How are reactions (1) and (2) actually combined?
a. A single enzyme actually catalyzes a 2 step rxn. for each AA & tRNA pair, such as his and tRNAhis. Having 2 steps (using same enzyme and matching same AA and same tRNA) increases accuracy as with proofreading by DNA polymerase.
b. Net result is to couple ATP hydrolysis and tRNA loading as follows (also on handout):
(a) ATP + AA → AMP~AA + PP
i(b) AA~AMP + tRNA → AA~tRNA + AMP
--------------------------------------------------------------------------------------------
net = ATP + AA + tRNA → AMP + PP
i + AA~tRNA = same as (1) + (2) above.As explained above, ΔGo for this is about zero, but pyrophosphatase pulls it as for DNA synthesis.
If you include action of pyrophosphatase, overall net = same as (1) + (2) + (3) above.c. Charging/loading reaction is very complex, but it serves 2 functions: accuracy/specificity and energy. 2 step part increases accuracy/specificity; overall rxn. hooks ATP hydrolysis to protein synthesis.
For a diagram, see Sadava fig. 14.13 (12.9) or Becker fig. 22-5.
d. Names of enzyme involved emphasize multiple functions of reactions above. Enzyme is called loading enzyme, activating/charging enzyme or aminoacyl-tRNA synthetase. Different names emphasize different job(s) of enzyme:
- loading enzyme -- ferries AA ('ferries the load') to mRNA
- Activating enzyme -- locks in energy for formation of peptide bond
- AA-tRNA synthase (or synthetase) -- does accurate matching of AA and tRNA; ensures specificity
Click here for animated protein synthesis (In Netscape >= 4, you can control the animation via the right mouse button. In Netscape 3, you can restart the animation with the Reload button and stop it at any point with the Stop button). This animation was made by a TA in this class. There are many more animations on the web. Go to Google and type in 'protein synthesis animation' for a whole collection. (Let Dr. M know if you find one that's really helpful.) Another animation is at http://highered.mcgraw-hill.com/olc/dl/120077/micro06.swf.
IV. Summary of How "RNA makes Protein"
A. How many different types of RNA are needed? To Review:
1. Role of mRNA. DNA codes for mRNA, and you use the mRNA to make protein. But is mRNA all you need? Of course not.
2. Also need tRNA and ribosomes (containing rRNA) as well as mRNA.
a. Where do tRNA and rRNA come from? All RNA's are encoded by DNA just like mRNA is. So there are genes for tRNA's and rRNA's on DNA. (Genes do not just code for proteins and respective mRNA's; genes also code for other RNA's needed along with mRNA to make proteins.)
b. tRNA and rRNA are not used as templates. When tRNA and rRNA genes are transcribed, the products fold up, associate with proteins if needed (for rRNA) and do their jobs. These RNA's are not translated -- they are agents that help in the translation of other RNA's (mRNA).
3. Different types of RNA have different half lives
a. tRNA and rRNA are relatively long-lived. Individual molecules last a long time and are used over and over before they are degraded.
b. Prokaryotic mRNA is relatively short-lived. mRNA's are constantly made, used for a short time, and degraded.
c. Eukaryotic mRNAs vary -- some but not all are short-lived; some are long-lived. Examples next term.
B. Does RNA alone make protein? No. We probably should say "Protein and RNA make protein"
You need enzymes, initiation factors (IF's) and elongation factors (EF's), ribosomal prot. etc. too, not just mRNA or just mRNA, tRNA and rRNA. (For all the details of IF's, EF's etc. see Becker fig. 22-10.) But you need protein to do everything; it's the RNA part that's unusual. Yes, this is a chicken and egg problem. It's why you need a cell -- with everything needed to make a protein -- to make (another) cell.
C. How many different types of the various RNA's are needed? Let's consider what it takes to make one polypeptide, and then what you have to change if you want to make a second, different peptide.
1. What does it take to make one peptide?
a. mRNA -- You need one kind to make one polypeptide.
b. rRNA -- You need several kinds (3-4) to make one ribosome; exact # depends on whether it's a eukaryotic or prokaryotic ribosome.
c. tRNA -- You need one complete set (to pick up all amino acids and read
all codons but stops). This is assuming the peptide you are making contains all
20 amino acids and the mRNA uses all possible codons. We calculated above that
will you need about 30 different kinds.
Note: question here is how
many different kinds do you need, not how many of each kind. If the same
codon occurs twice in a row, you will need two copies of the corresponding kind
of tRNA.
2. What if you want to make a different protein?
a. mRNA -- Will you need a different mRNA to make a second
(different) peptide? Yes (but see note at *) -- you need a unique
sequence of nucleotides (in the mRNA template) to make each unique peptide.
*Note: a single mRNA can sometimes carry several different sections,
each coding for a different peptide. In that case you could use a different
section of the same mRNA to make a different peptide.
Messenger RNAs that carry the information to make multiple
peptides are called "polycistronic mRNAs" and will be discussed when we get
to operons. Polycistronic mRNA's are common in prokaryotes but rare in
eukaryotes.
b. rRNA -- Once you have a complete set of rRNA's (and a ribosome), do you need a new set to make a second peptide? No, the same ribosome can read any message. (A real cell has many ribosomes, but all are the same.)
c. tRNA -- Once you have a complete set of tRNA's, will you need a new set to make a different peptide? No. The same set can be used over and over to made any number of different peptides. (A real cell has many molecules of each kind of tRNA, just as it has many ribosomes.)
3. Summary: mRNA is the software which is unique to the protein being made; tRNA & rRNA (& associated proteins) are the hardware that can be used to make any protein.
To review how many different RNA's it take to "make protein" do problems 7-14 & 7-17.
V. Mutations (if we don't get to this, we'll do it next time)
A. What are consequences of mistakes in macromolecular synthesis?
Protein Synthesis. If you make a mistake when lining up the AA, so what? 1 molecule of protein bad. No big deal as long as it doesn't happen too often.
Synthesis of tRNA
or rRNA. If you make a mistake lining up the
nucleotides in one molecule of tRNA or rRNA, results are similar.
Synthesis of mRNA. If
you make a mistake lining up the
nucleotides in mRNA, get a few bad protein molecules. Worse, but bearable. After this
molecule of mRNA gets thrown away, new mRNA and protein made will be ok.
Replication of DNA. If you make a mistake lining up the nucleotides in DNA, what then? Errors can get repaired, cells have enzymes for this. If not repaired, at next replication, one descendent gets a completely changed DNA and changed sequence is passed on forever. All new RNA and protein will be altered. So this is really serious, and this is what is meant by a mutation. (Mistakes in RNA and protein synthesis -- as long as the DNA is ok -- are not called mutations. They are called mistakes.)
B. How do mistakes in DNA synthesis occur?
Bases can mispair (if in wrong tautomeric form or damaged) -- See Sadava fig. 15.5 (12.20). DNA polymerase can slip relative to the template and add extra bases or leave some out. Proofreading keeps mispairing mistakes low but not zero. Repair enzymes correct some mistakes. (See below for why mutations are necessary.) After a mistake (putting in wrong base) occurs, other strand is still okay. But if DNA with mistake in one strand is replicated before mistake is corrected, one of daughter molecules will have two changed strands. (Other daughter molecule will be okay.)
A double stranded DNA molecule with one changed strand can still be corrected (by repair enzymes) but a molecule with two changed strands cannot be corrected. Once both strands are changed, the mutation is often said to be 'fixed' meaning 'permanent.' Note that in this context 'fixed' means the opposite of 'corrected.'
C. Definition/terminology of mutations See Becker Box 22B p. 694 (p. 700) or Sadava fig. 15.2 (chap. 12.6 p. 275-276).
1. Mistakes vs Mutations. Mistakes in RNA or protein synthesis are called mistakes, but mistakes in DNA synthesis (that are not corrected) are called mutations. Anything that changes the DNA is called a mutation, and an organism with a mutation is called a mutant. The normal or starting (or standard) organism is often called "wild type." A change in the RNA or protein that does not affect the DNA is not called a mutation.
2. Types of Mutations -- Terminology See Sadava fig. 15.2 (ch. 12.6) or Becker Box 22B
a. Substitution = change in base(s).
b. Deletion/insertion = removal or addition of base(s).
c. Frameshift. An insertion/deletion of 1 or 2 bases (in a coding region) is called a frameshift because mRNA with such a mutation is misread in the wrong "reading frame" (wrong groups of three nucleotides) all the way to the end of the gene (or until ribosome reaches a stop codon). Notice the drastic difference in effects between substitutions and frameshifts.
d. Nonsense vs Mis-sense. A mutation that generates a stop codon is sometimes called a "nonsense" mutation; one that changes one amino acid to another is called a "mis-sense" mutation.
3. Phenotype and genotype
The state of the DNA is known as the genotype; the observable properties of the organism are known as the phenotype. A mutation changes the genotype, but may or may not change the phenotype. See recitation problems #8.
D. Why are mutations important?
1. Source of evolutionary diversity -- source of all variation in phenotype for selection to act on; why there are different species (& why we're here at all). This is good overall, but not good for us when it's HIV or flu or any other infectious agent that's mutating.
2. Source of individual (& nonfunctional) diversity. Mutation leads to variations in noncoding DNA. This has little or no functional consequences, but the variations come in handy for tracing evolutionary lines of descent and making identifications. (This is the basis of all forensic ID's.) Variations that do not affect phenotype persist because there is no selection for or against any particular version. (Individuals that carry any particular mutation are not at any reproductive advantage or disadvantage.)
3. Cause inherited diseases like hemophilia, Tay Sachs, etc. (Can cause cancer in somatic cells.) To keep advantages of (1) and avoid disadvantages of (3), organisms keep the mutation level low but nonzero by extensive editing, repair, etc. of DNA
4. Mutations are a very useful tool for figuring out how things work.
Studying effects of frameshifts allowed us to start to crack the genetic code -- see prob book, recitation problems and texts (for details see 'frameshift mutations' in Becker)
Allows us to knock out one protein at a time and see what happens -- implies what function of protein was in first place. (As in figuring out pathways in prob. book.) Same affect can often be achieved with RNAi (or antisense), or with drugs.
Note: In this course, we often tell you how it works first and then give you mutations to test your understanding. Historically, it usually works in reverse -- mutations are studied first and details of 'how it works' are figured out from analyzing the mutants. For example, the genetic code was partially 'cracked' by looking at mutations and seeing how changes in the DNA correlated to changes in the corresponding protein. (It took biochemistry to finish the job.) See texts for details.
To review mutations, see recitation problems #8 and problem 7-22. (7-23, 7-24 & 7-26 also deal with mutations.)
© Copyright 2010 Deborah Mowshowitz and Lawrence Chasin Department of Biological Sciences Columbia University New York, NY .