The Covalent Bond
Classification Method:
A New Approach to the
Formal Classification of
Covalent Compounds of the Elements
Malcolm L. H. Green
J. Organomet. Chem. 1995, 500, 127-148.
Introduction
Covalent molecules are
often described in terms of the oxidation state formalism in which a charge is
assigned to the atom of interest.
While the oxidation state concept has proven to be of use in the
traditional coordination chemistry with simple ligands, e.g. Cl– and NH3, it has
become evident that this concept is of limited utility in organometallic and
modern coordination chemistry because of the more complex nature of the ligands
involved. For example, the
cycloheptatrienyl (C7H7) ligand has been assigned charges
of +1, –1, and –3; as such, it is evident that the oxidation state
of the metal has little meaning in complexes with such ligands. In this regard, a recent IUPAC article
concludes that it is inappropriate to assign oxidation numbers with respect to
the nomenclature of organometallic compounds, viz: “As oxidation numbers cannot be assigned
unambigously to many organometallic compounds, no formal oxidation numbers will
be attributed to the central atoms in the following section on organometallic
nomenclature.” 1999, 71, 1557-1585).
Also criticizing the use of
oxidation states, Seddon and Seddon have written: “…the oxidation state concept can be thought of as the Dewey
Decimal Classification of inorganic chemistry – if the rules are applied,
a number is obtained”. But Seddon
and Seddon continue: “Does oxidation state have a chemical significance? A number is always obtained –
does it mean anything?” (The Chemistry of Ruthenium, Seddon, E. A.; Seddon, K. R. Elsevier, New York,
1984; Chapter 2.)
In addition to problems
associated with assignment of oxidation number, the assignment of “coordination
number” is often ambiguous because the term is interpreted with more than one
meaning in the literature. For
example, what is the coordination number of chromium in (h6‑C6H6)2Cr?
Common answers include 2, 6 and
12, depending on one’s notion of “coordination number”.
The problems associated
with classifying molecules by oxidation number and coordination number stem
from the application of a classification system to a set of molecules for which
it is not appropriate. In order to
surmount problems of the types described above, Malcolm Green introduced an
innovative method for the formal classification of covalent compounds (Green,
M. L. H. J. Organomet. Chem. 1995, 500,
127-148). The principal advantage
of the so‑called “Covalent Bond Classification (CBC) Method” is that it
was specifically designed for covalent molecules.
In essence, the CBC method
seeks to classify a molecule according to the nature of the ligands around the
central element of interest. The
method is based on the notion that there are three basic types of interaction
by which a ligand may bond to a metal center and the ligand is classified
according to the nature and number of these interactions.
The three basic types of
interaction are represented by the symbols L, X, and Z, which correspond
respectively to 2-electron, 1-electron and 0-electron neutral ligands and are clearly differentiated according to
a molecular orbital representation of the bonding.
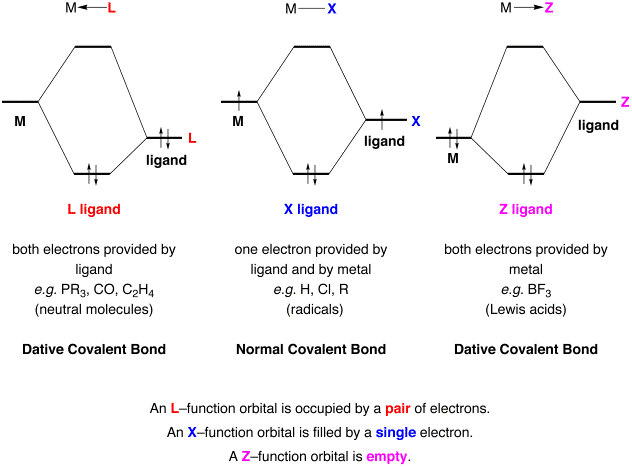
An L‑function
ligand is one which interacts with a metal center via a dative covalent bond (i.e. a coordinate bond), in which both electrons are
donated by the L ligand. As such,
an L‑function ligand donates two electrons to a metal center. Since the metal uses no electrons in
forming the M–L bond, an L‑function ligand does not influence the
valence of a metal center. Simple
examples of L‑type ligands include R3P, R2O, and
CO, i.e. donor molecules that
have lone pairs (Lewis bases).
An X‑function
ligand is one which interacts
with a metal center via a normal
2‑electron covalent bond, composed of 1 electron from the metal and 1
electron from the X ligand. As
such, an X‑function ligand donates one electron to a metal center. Since the metal uses one electron in
forming the M–X bond, each X‑function ligand raises the valence of
the metal center by one unit.
Simple examples of X‑type ligands include H and CH3, i.e. radicals.
A Z‑function
ligand also interacts with a
metal center via a dative
covalent bond, but differs from the L‑function in that both electrons are
donated by the metal rather than the ligand. As such, a Z‑function ligand donates zero electrons to
a metal center. Since the metal
uses two electrons in forming the M–Z bond, a Z‑function ligand
raises the valence of the metal center by two units. Simple examples of Z‑type ligands include BF3,
BR3 and AlR3, i.e. molecules that have a vacant orbital (Lewis acids).
More important than merely
referring to the number
of electrons involved in the bonding is the fact that the types of interaction
are differentiated according to the nature of the molecular orbital interaction (see
above). It is, therefore, apparent
that the CBC method is of much more relevance to classifying and providing
insight into the nature of covalent
organometallic molecules than are methods based on (i) oxidation number (which
merely hypothetically decomposes a molecule into its constituent ions) and (ii)
electron count (that focuses only on the number of electrons, regardless of
their origin).
A given ligand may have one
or more of the above functions. As
such, the ligand may be classified as [XxZz],
where l, x, and z
are the respective number of L, X, and Z functionalities. For example, the h6‑benzene
ligand is classified as [L3], with the three L functionalities
corresponding to the three “olefinic” moieties (see below). Likewise, the h5‑cyclopentadienyl
ligand is classified as [L2X], with the two L functionalities
corresponding to the two “olefinic” fragments while the X functionality
corresponds to the CH “radical” portion of the resonance structure.
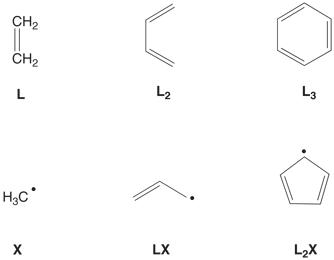
At a more fundamental level
than merely relating to the number of electrons a ligand donates, however, the
[LlXxZz] classification refers to the nature of the frontier orbitals of the neutral ligand, as illustrated for the Cn–symmetric CnHn
ligands (see below).
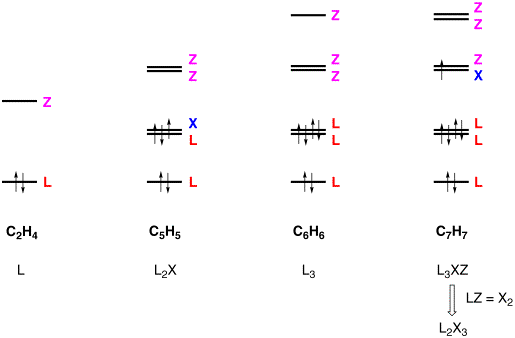
For example, the three
highest energy occupied orbitals of the C5–symmetric C5H5 radical
comprise a pair of doubly degenerate orbitals (HOMO) and a nondegenerate
orbital (HOMO–1). The
HOMO–1 orbital is fully occupied and corresponds to an L function, while
the HOMO is occupied by three electrons and corresponds to an L and an X
function. As such, C5H5
is classified as an [L2X] ligand. The three highest energy occupied orbitals of C6H6
also comprise a pair of doubly degenerate orbitals (HOMO) and a nondegenerate
orbital (HOMO–1), but since these are all occupied, C6H6
is classified as an L3 ligand.
An interesting situation arises, however, with C7–symmetric C7H7
because the HOMO is a singly occupied doubly degenerate orbital. As such, the HOMO is an XZ combination. Coupled with the L2 nature
of the fully occupied doubly degenerate HOMO–1 orbital, and the L nature
of the HOMO–2 orbital, the C7H7 ligand is
classified as L3XZ; however, this classification reduces to L2X3
because an LZ combination is equivalent to that of an X2
combination. In essence, one may
view the valence state of the C7H7 ligand to have three
unpaired electrons, in much the same way that carbon has a sp3
valence state with four unpaired electrons when it combines to form a
tetrahedral compound.
The Equivalent Neutral
Class
Once all the ligands about
a metal center have been classified as described above, the molecule itself is
classified as [MLlXxZz]Q± by summing all the L‑, X‑, and Z‑
functionalities, as illustrated below for some tungsten complexes.
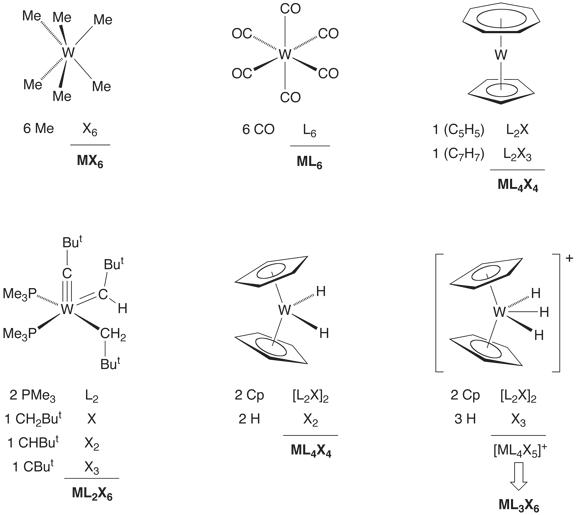
For example, Cp2WH2
is classifed as [ML4X4] since Cp ![]() [L2X]
and H
[L2X]
and H ![]() [X].
Correspondingly, [Cp2WH3]+ would be
classified as [ML4X5]+. However, in order to allow for
comparisons between molecules that have different charges, it is useful to
reduce the [MLlXxZz]Q± assignment to its “equivalent neutral class”, which is
essentially the classification that would be obtained if the Q±
charge were to be localized on the ligand and not on the metal center.
[X].
Correspondingly, [Cp2WH3]+ would be
classified as [ML4X5]+. However, in order to allow for
comparisons between molecules that have different charges, it is useful to
reduce the [MLlXxZz]Q± assignment to its “equivalent neutral class”, which is
essentially the classification that would be obtained if the Q±
charge were to be localized on the ligand and not on the metal center.
For cations, the
transformations are: (i) L+ ![]() X, i.e. a cationic 2‑electron donor is equivalent to
a neutral 1‑electron donor, and (ii) X+
X, i.e. a cationic 2‑electron donor is equivalent to
a neutral 1‑electron donor, and (ii) X+ ![]() Z, i.e. a cationic 1‑electron ligand is equivalent to
a neutral 0–electron ligand.
For anions, the most commonly encountered transformations are: (i) X–
Z, i.e. a cationic 1‑electron ligand is equivalent to
a neutral 0–electron ligand.
For anions, the most commonly encountered transformations are: (i) X– ![]() L, i.e. an anionic 1‑electron donor is equivalent to
a neutral 2-electron donor and (ii)
L–
L, i.e. an anionic 1‑electron donor is equivalent to
a neutral 2-electron donor and (ii)
L– ![]() LX, i.e. an anionic 2‑electron donor is equivalent to
a 3‑electron donor. It is
important to emphasize that the latter two transformations should be applied
sequentially, i.e. a negative
charge is only placed on an L–function if there is no
X–function. Finally, if the
derived classification after performing the above transformations contains both
an L and a Z function, the classification is reduced further by using the
transformation LZ = X2.
LX, i.e. an anionic 2‑electron donor is equivalent to
a 3‑electron donor. It is
important to emphasize that the latter two transformations should be applied
sequentially, i.e. a negative
charge is only placed on an L–function if there is no
X–function. Finally, if the
derived classification after performing the above transformations contains both
an L and a Z function, the classification is reduced further by using the
transformation LZ = X2.
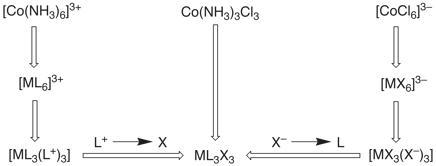
A consideration of some
simple compounds serves to indicate the rationale for describing a molecule in terms
of its equivalent neutral class.
For example, the cationic, neutral, and anionic octahedral Co(III)
species [Co(NH3)6]3+, [Co(NH3)3Cl3], and
[CoCl6]3_,
which are related by the formal substitution of Cl– by NH3,
each belong to the same fundamental molecular class, i.e. [ML3X3] (see below). Thus, even though the compounds have
different charges, the CBC method indicates that the three molecules belong to
the same class of compound.
The [MLlXxZz] classification, electron number, valence and
ligand bond number
Once
the [MLlXxZz] classification of a molecule is known, it is a simple matter to
extract other useful information pertaining to the nature of a molecule,
including the electron count, valence, and ligand bond number (Table 1).
Table 1. Definitions pertaining to the CBC method.
|
Symbol |
Definition |
|
L |
2–electron donor function |
|
l |
number of L functions |
|
X |
1–electron donor function |
|
x |
number of X functions |
|
Z |
0–electron donor function |
|
z |
number of Z functions |
|
m |
number of electrons on neutral metal |
|
VN |
valence number VN = x + 2z |
|
LBN |
ligand bond number l + x + z |
|
EN |
electron count m + 2l + x |
|
dn |
number of electrons in
“nonbonding” metal orbitals n = m – x – 2z = m – VN |
For
example, the electron number (EN) of the metal in [MLlXxZz], i.e. the electron count, is given by EN = m + 2l
+ x, where m is the number of valence electrons on the neutral
metal atom.
The
valence number (VN) of the metal center, i.e. the number of electrons that the metal uses in
bonding, is VN = x + 2z. In
most organotransition metal complexes, the number of Z ligands in the
equivalent neutral class is zero.
As such, the valence number is typically equal to x, i.e.
the number of one-electron donor X‑ligands. The value of the dn configuration is given by n =
m – x – 2z = m – VN.
Finally, the ligand bond
number (LBN) represents the effective total number of ligand functions surrounding M, and is defined as LBN = l + x
+ z. While not defined as the coordination number, it is
pertinent to note how the ligand bond number as defined by l + x
+ z gives the value that
organometallic chemists “want” the coordination number to be in many
compounds. For example, both (h5–C5H5)2Cr
and (h6–C6H6)2Cr (i.e. ML4X2 and ML6,
respectively) have a ligand bond number of six, even though the classical
definition of coordination number gives values of 10 and 12, respectively. Furthermore, it is noteworthy that the
ligand bond number reduces to the classical definition of coordination number
when the ligands are “simple”, i.e. monofunctional ligands that coordinate to the metal using a single
orbital, e.g. H and CH3.
MLX Plots
Since the [MLlXxZz]
classification contains information that relates to the electron count, the
valence and ligand bond number, it provides a greater dimension for classifying
compounds than methods based on either electron count or oxidation number. The [MLlXxZz]
classification may be represented as a function of the electron count and
valence number of the metal in diagrams are often simply referred to as “MLX
plots”. An illustration of an MLX
plot is provided by the example for organometallic compounds of iron is shown
below (data compiled by Cary Zachmanoglou from compounds listed in the Dictionary
of Organometallic Compounds); MLX
plots for other elements are presented at the end of this article.
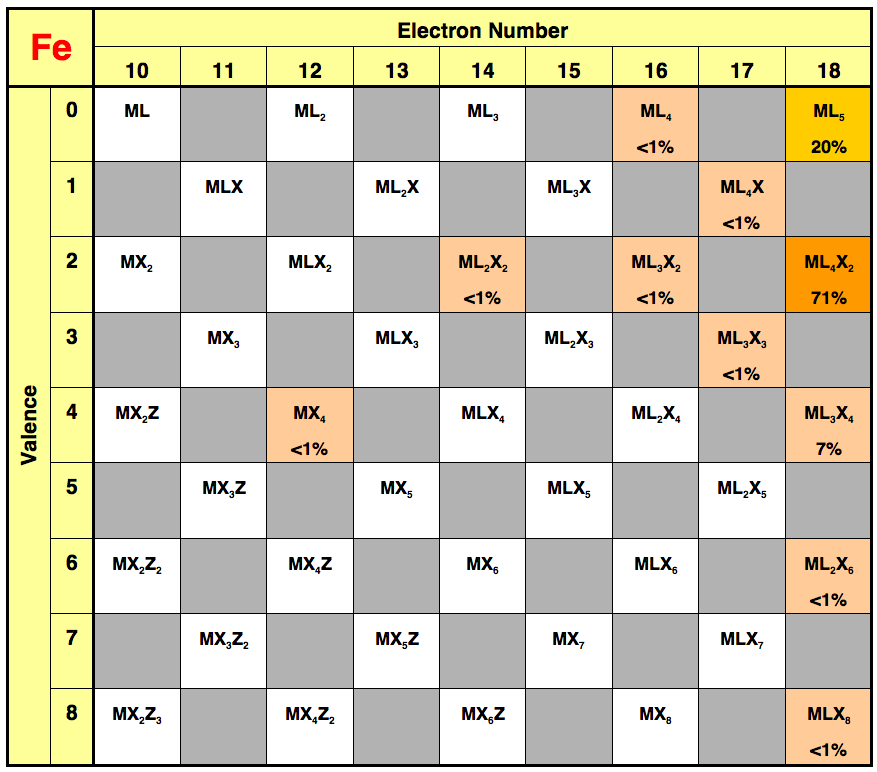
For example, while
consideration of the electron count for iron compounds results in the
conclusion that the organometallic chemistry of iron is dominated by
18–electron molecules, i.e.
a single class of molecules, consideration of the MLX plot shows that this
class of molecule can be further conveniently divided into additional
classes. Specifically,
18–electron iron complexes belong to [ML5], [ML4X2],
and [ML3X4] classes, representatives of which are Fe(CO)5,
Cp2Fe, and [CpFe(CO)(m–CO)]2.
MLX plots are a
characteristic of each element and are provided at the end of this article for
organometallic compounds of Groups 3 – 10 transition metals (Parkin, G.
in Comprehensive Organometallic Chemistry III, Volume 1, Chapter 1; Crabtree, R. H. and Mingos,
D. M. P. (Eds), Elsevier, Oxford, 2006).
In general, each element favors one or several [MLlXxZz] classes
and the three most common for each element are summarized below.
|
Group 3 |
Group 4 |
Group 5 |
Group 6 |
Group 7 |
Group 8 |
Group 9 |
Group 10 |
|
ScL4X3
(36%) ScL5X3
(33%) ScL3X3
(10%) |
TiL4X4
(49%) TiL5X3
(9%) TiL2X4
(7%) |
VL6X (22%) VL4X4
(16%) VL4X3
(14%) |
CrL6 (48%) CrL5X2
(24%) CrL4X4
(7%) |
MnL5X (79%) MnL4X3
(12%) MnL3X5
(1%) |
FeL4X2
(69%) FeL5 (20%) FeL3X4
(7%) |
CoL3X3
(54%) CoL4X (34%) CoL2X5
(4%) |
NiL2X2
(33%) NiL3X2
(26%) NiL4 (16%) |
|
YL5X3
(37%) YL6X3
(22%) YL4X3
(19%) |
ZrL4X4
(55%) ZrL5X4
(25%) ZrL6X2
(6%) |
NbL5X3
(32%) NbL6X (17%) NbL4X5
(15%) |
MoL5X2
(40%) MoL4X4
(25%) MoL6 (19%) |
TcL5X (75%) TcL4X3
(14%) TcL3X5
(2%) |
RuL4X2
(79%) RuL3X4
(9%) RuL5 (8%) |
RhL3X (41%) RhL3X3
(27%) RhL4X (22%) |
PdL2X2
(81%) PdL3X2
(9%) PdL3 (4%) |
|
LaL4X3
(31%) LaL6X3
(22%) LaL3X3
(17%) |
HfL4X4
(58%) HfL6X2
(11%) HfL5X4
(8%) |
TaL2X5
(23%) TaL4X5
(15%) TaL5X3
(14%) |
WL5X2
(34%) WL4X4
(27%) WL6 (15%) |
ReL5X (49%) ReL4X3
(29%) ReL3X5
(4%) |
OsL4X2
(83%) OsL5 (8%) OsL3X4 (7%) |
IrL3X3
(47%) IrL3X (26%) IrL4X (20%) |
PtL2X2
(69%) PtL2X4
(11%) PtL3 (9%) |
Trends elucidated from
MLX plots
By summarizing a vast
quantity of factual information, the MLX plot reveals important characteristics
of the chemistry of the element under consideration and the information
embodied in an MLX plot enables a variety of periodic trends to be established
by comparing the distributions for the elements.
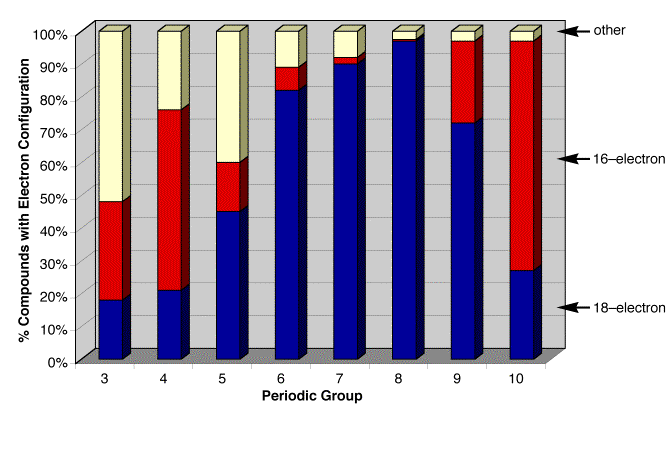
(i) Electron count
Since the electron count
for a molecule of class [MLlXxZz] is given by EN = m + 2l
+ x, it is a simple matter to
use the [MLlXxZz] classification to evaluate the distribution of molecules according to
a specific electron count. For
example, the data indicate that
“18‑electron rule” is most closely obeyed for the middle portion
of the transition series (Groups 6 – 8), as illustrated by the blue
bars. Both the earlier and later
transition metals exhibit many deviations from this rule.
(ii) Valence
With respect to the distribution
of valence as a function of group, it is evident that the occurrence of the
group valence is common up to Group 5, after which the two lowest valence
states with an even dn configuration become prevalent (see
below).
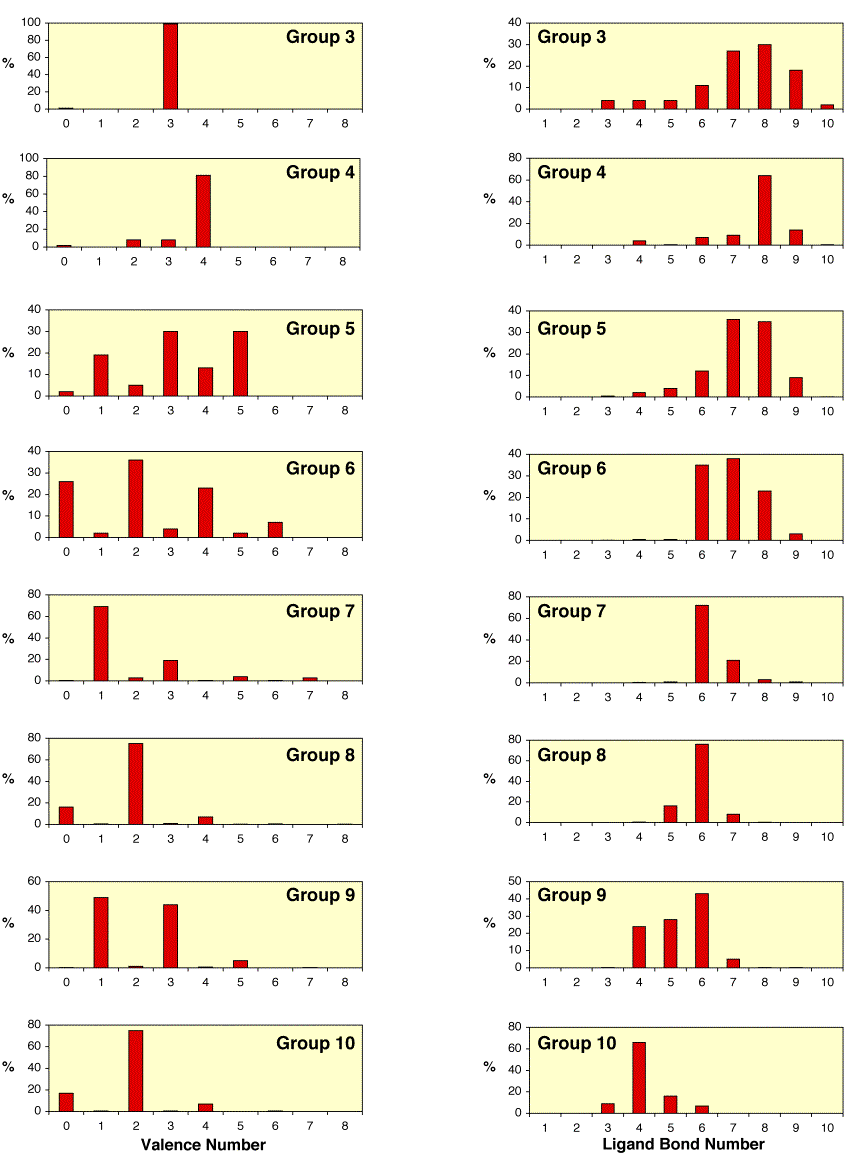
(iii) Ligand bond number
The ligand bond number (cf.
“coordination number”) decreases
smoothly upon passing from Group 3 to Group 10 (see above). Specifically, the most common ligand
bond number decreases from 8 for Group 3 to a value of 4 for Group 10.
Summary
In summary, the CBC method
is based on an elementary molecular orbital analysis of metal–ligand
bonding interactions and a molecule is described in terms of the representation
MLlXxZz where Ll, Xx and Zz refer to the number of 2–electron,
1–electron, and 0–electron donor functions. By embodying the electron count (m + 2l
+ x), the valence of the metal (x + 2z),
the ligand bond number (l + x + z),
and the dn configuration (n = m – x – 2z), the MLlXxZz classification
affords much more information than that provided by the oxidation number. Furthermore, by identifying the
different types of metal–ligand bonding interactions, the MLlXxZz classification
describes the nature of the metal in the molecule of interest, while the oxidation number merely
describes the charge on the metal after all ligands have been removed (!). Finally, since there are several different
methods used to assign oxidation numbers, there is often ambiguity in the
derived values. These problems are
exacerbated for organometallic compounds and, as such, the MLlXxZz classification
provides a much more useful method of classification for these molecules.
MLX plots for
organometallic compounds of the transition metals of Groups 3 – 10 and
distributions of electron count, valence and ligand bond number are available
here. For further discussion of
the CBC method, see: Parkin, G. in
Comprehensive Organometallic Chemistry III, Volume 1, Chapter 1; Crabtree, R. H. and Mingos, D. M. P. (Eds),
Elsevier, Oxford, 2006.
|
|||
