|
The members of the TERML are committed to the discovery of the natural development of cells and tissues, and to the engineering of cells, tissues and organs to benefit the well-being of mankind.
Stem Cells
What are stem cells?
Stem cells have two essential properties (Alhadlaq and Mao, 2004; Mao, 2005; Marion and Mao, 2006):
- Stem cells replicate multiple generations into offspring cells which in turn can replicate;
- A single population of stem cells can differentiate into multiple tissue-forming cells. For example mesenchymal stem cells can differentiate into osteoblasts, chondrocytes and adipocytes that form bone, cartilage and fat respectively.
How are stem cells tracked?
Stem cells can be tracked with organic dyes, GFP-infected virii and nanoparticles.
We provide a wide spectrum of training opportunities for undergraduate students, graduate students and postdoctoral fellows in the following areas: Stem Cell Research and Tissue Engineering, Nanobiology, and Mechanobiological Regulation of Skeletal Growth.
Tissue engineering is predicted by Science, Nature and Time magazines to be the next area of breakthrough. Our research projects are currently funded through grants from National Institutes of Health and other sources. We are looking for self-motivated individuals who would like to have a challenging and productive career in science. We currently have a team of postdoctoral fellows, graduate students (MS and PhD) and undergraduate students with diverse backgrounds, such as biology, engineering (bioengineering, tissue engineering, chemical engineering and mechanical engineering), material science, as well as medicine and dentistry.
Stem Cell Research, Tissue Engineering and Regenerative Medicine
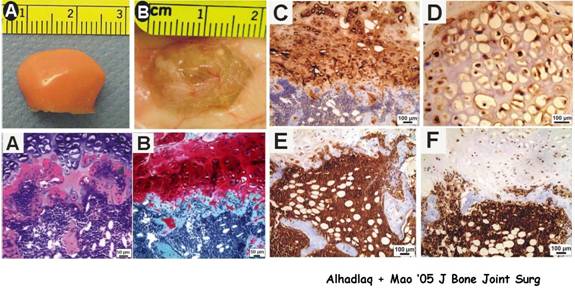
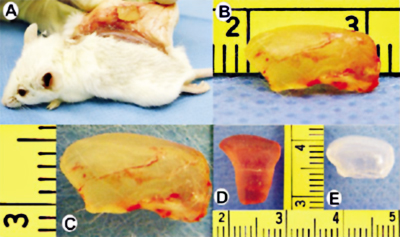
Differentiation of adult mesenchymal stem cells into osteogenic, chondrogenic and odontogenic cells and subsequent modulation of biomineralization involves cell culture, the use of growth factors and atomic force microscopy to study osteogenesis (bone), chondrogenesis (cartilage) and odontogenesis (tooth). This approach can be characterized as molecular biology and nanomechanics. This approach also involves reconstruction of 3-D scaffolding polymers for the repair of critical size defects. Scaffolding materials will be designed to facilitate the healing of critical size defects with osteoinductive and osteoconductive intervention, such as growth factors and mesenchymal stem cells. The outcome of skeletal healing is studied by computerized microscopic histomorphometry, cell counting, in situ hybridization, and nanoscopic analysis with atomic force microscopy. The ultimate goal of this approach is to synthesize skeletal materials for biomimetic regeneration of bone, cartilage and teeth.
Nanobiology
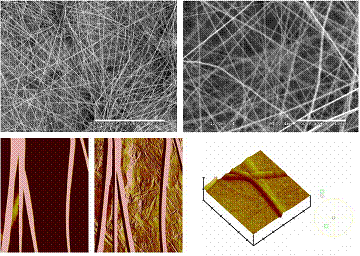 Using atomic force microscopy to study collagen, mineralizing bone, hyaline cartilage, fibrocartilage (e.g., intervertebral discs) and implant-bone interface. Nanoindentation tests are applied to determine material properties of these skeletal tissues. Atomic force microscopy is an advanced imaging tool that allows scientists to not only visualize structures in the nanometer ranges (1 nm = 1 millionth of 1 mm), but also to measure intermolecular forces. Using atomic force microscopy to study collagen, mineralizing bone, hyaline cartilage, fibrocartilage (e.g., intervertebral discs) and implant-bone interface. Nanoindentation tests are applied to determine material properties of these skeletal tissues. Atomic force microscopy is an advanced imaging tool that allows scientists to not only visualize structures in the nanometer ranges (1 nm = 1 millionth of 1 mm), but also to measure intermolecular forces.
Nanofibers are fine material filaments fabricated from materials science and engineering approaches. Nanofibers can be used to simulate fibrous proteins such as collagen in the process of tissue engineering. We have demonstrated that nanofibers can accommodate the proliferation and differentiation of human stem cells.
Quantum dots (QDs) are nanoparticle crystals in the size range of 2-10 nm. We have demonstrated that bioconjugated QDs can be used to labeling human stem cells during proliferation and multi-lineage differentiation into osteoblasts, adipocytes and chondrocytes.
Mechanobiological Regulation of Skeletal Growth
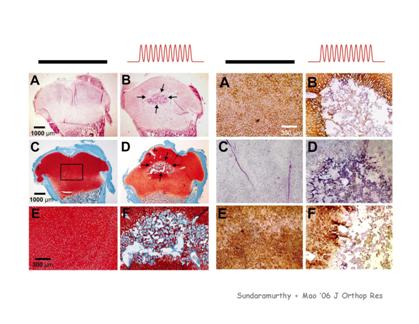 The computerized delivery of biomechanical stimuli both in vivo (animal models) and in vitro (organ culture) to induce osteogenesis and chondrogenesis. Biomechanical stimuli of different magnitudes and frequencies are delivered to animal models and tissue explants. Bioreactors are used to mimic biological environments for organ culture. The outcome of biomechanical stimuli is quantified by computerized microscopic histomorphometry, cell counting, in situ hybridization, and nanoscopic analysis with atomic force microscopy. In addition, PCR and gel electrophoresis are used to determine the expression of several molecules and genes of interest in the extracellular matrix of bone and cartilage. The computerized delivery of biomechanical stimuli both in vivo (animal models) and in vitro (organ culture) to induce osteogenesis and chondrogenesis. Biomechanical stimuli of different magnitudes and frequencies are delivered to animal models and tissue explants. Bioreactors are used to mimic biological environments for organ culture. The outcome of biomechanical stimuli is quantified by computerized microscopic histomorphometry, cell counting, in situ hybridization, and nanoscopic analysis with atomic force microscopy. In addition, PCR and gel electrophoresis are used to determine the expression of several molecules and genes of interest in the extracellular matrix of bone and cartilage.
|

