An Interdisciplinary Collaboration between
Engineering and Medicine.
|
Mark Borden,
PhD, Asst. Professor of Chemical Engineering Jessica
Kandel, MD, Assoc. Professor of Surgery Darrell Yamashiro,
MD/PhD, Assoc. Professor of Pediatrics |
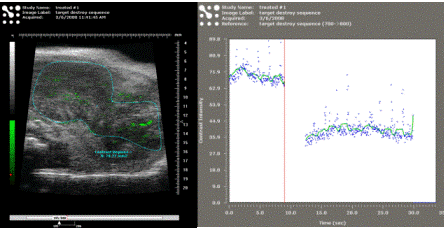
An ultrasound molecluar imaging scan showing the extent of
αVβ3 integrin (a marker for angiogenesis) exrpession in the tumor
microenvironment. Left: the image
shows microbubble signals in green.
Right: the plot shows a signal intensity change after a destruction
pulse to deliniate signal from targeted vs. free microbubbles. |
Mark Borden, PhD
Dr. Borden is an Assistant Professor of Chemical Engineering at Columbia
University. His research entails the
design and engineering of novel microbubble constructs for ultrasound molecular
imaging and targeted drug delivery. Dr.
Borden is leading the implementation of microbubble technology to assess tumor
vasculature via ultrasound.
Jessica Kandel, MD
Dr. Kandel is an Associate Professor of
Surgery. Her research specializes in the
regulation of angiogenesis in pediatric solid tumors, including Wilms Tumor and
neuro-blastoma. Dr. Kandel is leading
the development of animal models and ex vivo techniques and translation of
results to clinical practice.
|
|
|
|
Darrell Yamashiro, MD/PhD
Dr.
Yamashiro is an Associate Professor of Pediatrics and Pathology and Cell
Biology (in Surgery). He is the co-director
(with Dr. Kandel) of the Pediatric Tumor Biology Laboratory, which focuses on
the role of angiogenesis in promoting the growth and metastasis of pediatric
solid tumors.
Dr. Sirsi is a Postdoctoral Research
Scientist working with Dr. Borden. He is
the key liaison between the laboratories and is responsible for the execution
of microbubble development and ultrasound imaging and therapy experiments.
Combined Molecular/Ultrasound Analysis
of Tumor Response to VEGF
Funding: NIH/NCI R21 CA 139173
PI: Borden and Kandel
Our ultimate goal is to develop
an innovative ultrasound technique to monitor and guide anti-angiogenic therapy
for children with clinically aggressive Wilms tumors (WT). We are developing
this technology, which includes molecularly targeted microbubble contrast
agents and sophisticated imaging methods, while exploring tumor vascular
changes during initial and chronic blockade of vascular endothelial growth
factor (VEGF). We hypothesize that molecular changes in WT angiogenesis can be 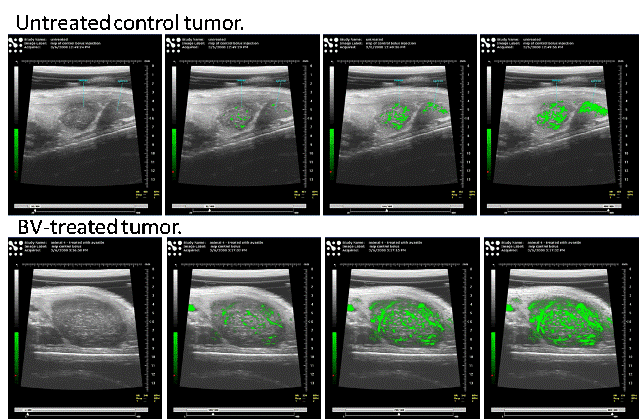 correlated
with vascular changes revealed by noninvasive ultrasound.
correlated
with vascular changes revealed by noninvasive ultrasound.
Children with unfavorable histology
Wilms tumor (WT) and metastatic disease continue to experience high mortality
rates. These patients urgently require new therapies. Drs. Kandel and Yamashiro
have recently reported Phase I data indicating excellent tolerance of the
anti-vascular endothelial growth factor (VEGF) antibody bevacizumab (BV) in
refractory pediatric tumors. Because this therapy has been validated in adult
cancers, it may provide an attractive option for patients with aggressive WT;
however, methods of assessing tumor response clinically are lacking. This is a
particularly critical issue for pediatric cancer patients, in whom long-term
tumor control is the goal. In previous studies, Drs. Kandel and Yamashiro
reported that experimental WT were initially strikingly suppressed by VEGF
inhibitors. Yet consistent with clinical observations that adults treated with
BV virtually all progress, they found that even highly responsive xenografts
resumed growth if treatment was sustained. The mechanism of resistance to VEGF
blockade is poorly understood, and clinical endpoints of resistance remain
undefined. Emerging data suggest that tumors subjected to VEGF inhibition
exhibit features of ischemic injury, including induction of damage response
pathways and vessel remodeling. Further, distinct changes in gene expression,
vascular assembly, and perfusion occur both acutely and chronically. For
example, Drs. Kandel and Yamashiro have previously reported that VEGF
inhibition can cause striking loss of branching vasculature and ischemia by 24
hours, whereas long-term blockade results in vessel remodeling, recovery of
flow, and tumor progression. Key molecular markers of the response to vessel injury
include members of gene families that are essential to angiogenesis, including
integrins (alphaVbeta3), VEGF receptors (VEGFR-1 and -2), and Notch family
members (Jagged-1), and mediators of the response to hypoxia (such as COX-2).
High frequency ultrasound is an
emerging technology that can provide rapid and longitudinal assessment of the
anatomic, functional, and physiological response of WT vasculature to VEGF
inhibitors. The excellent sensitivity of newly available commercial scanners to
sonographic contrast agents (microbubbles) echo-signatures facilitates
visualization of vessel architecture, quantification of blood flow, and
molecular imaging of endothelial biomarkers in solid tumors. Yet this
technology is still in its infancy, and further development of long-circulating
and targeted microbubbles is critical for realizing its full potential as a
means of evaluating dynamic changes in vessel structure and function. In
particular, it is essential to develop a platform suitable for clinical point-of-care
use. In these studies, we will investigate vascular remodeling during VEGF
blockade using high frequency ultrasound, in the specific context of
experimental WT, and using novel microbubble tools and ultrasound imaging
techniques. Our goal in these studies is to relate acute and chronic molecular
changes in WT angiogenesis with highly quantitative and sensitive architectural
and flow characteristics and vascular biomarker expression patterns revealed by
ultrasound.
|
|
|
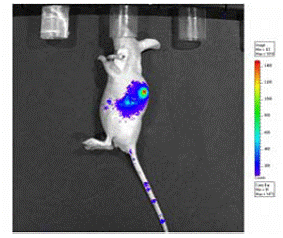
Xenogen image of mouse
tumor transfected with a bioluminescent reporter gene. |
Novel microbubble-based gene delivery
vehicles targeting solid tumors
Funding: St.
Baldrick’s Foundation
PI: Yamashiro
Novel
microbubble-based gene delivery vehicles will be engineered to provide an efficient
and selective means of transfecting target tumors with DNA. The research will not
only result in advanced gene delivery technology, but will also better characterize
the underlying mechanisms of ultrasound-microbubble gene delivery. Together,
these studies may move this concept and technology rapidly toward clinical
treatment of children with cancer.
Previous
workers have used microbubbles and ultrasound to deliver genetic material, in
an effort to bypass the need for viral vectors. By exploiting the ability of
ultrasound to precisely control microbubble destruction, researchers have
demonstrated highly specific tissue targeting of proteins and plasmids to the
heart (Shohet et al., 2000; Mayer and Bekeredjian, 2008), tumors (Nie et al.,
2008), and other tissues (Unger et al., 2004; Chen et al., 2006). Although
promising, the current state of this novel gene delivery method has yet to
achieve efficient transfection within a clinically practical microbubble
concentration. Microbubbles bearing genetic material, e.g. by charge-coupling
of DNA to the cationic surface, can be introduced into cells by transient
sonoporation. DNA-loaded microbubbles are injected intravenously, and high pressure,
low-frequency ultrasound is then applied to the region of interest to destroy
microbubbles as they pass through the local microcirculation, producing a
site-specific concentration of the gene. Ultrasound-induced oscillation of the
gas core of the microbubble creates pores in endothelium and surrounding cell
membranes, through which genetic material may pass to enter cytoplasm. Larger
molecules, such as DNA plasmids, may also enter cells via an endocytic pathway.
In addition, microbubble-based agents can be observed by ultrasound,
demonstrating localization and concentration of injected materials.
However,
a challenge is posed by the finite surface area of current microbubble
vehicles, which limits loading capacity (nucleic acids are insoluble in the gas
phase and thus cannot be encapsulated). For example, current loading capacity
of lipid-coated microbubbles is approximately 80 μm2 for a 5-μm diameter microbubble. Considering
a “hit-and-stick” adsorption model, the surface density is approximately 0.0001
pg/μm2 for a 10 kbp DNA plasmid,
resulting in an estimated maximum loading density of ~0.01 pg/microbubble.
Thus, a method is needed to condense DNA and increase the total available
surface area of the microbubble. One technique we have previously used to
increase loading capacity is by layer-by-layer (LbL) assembly of a polyelectrolyte
multilayer (PEM) composed of DNA and a biocompatible polycation (Borden et al.,
2007). Unfortunately, although this led
to excellent loading capacity, the bioavailability was poor, leading to
insufficient transfection efficiency. In
the current research, we are developing an alternative strategy to improve DNA
packaging for enhanced release from the microbubble shell and subsequent intracellular
trafficking.
Copyright Columbia University