 3. Help! |
 3. Help! |
In 1985 the curators of the soon-to-open New Mexico Museum of Natural History were each invited to present a short seminar at nearby Los Alamos National Laboratory. Expecting that the scientists attending my presentation would have little interest in the anatomy of Ice Age glyptodonts or the biogeography of Miocene sharks in the Caribbean (two of my research projects to date), I chose instead to discuss local paleontology. More important, I decided to share two problems. These were not paleontological problems in the usual sense, but problems that had bothered me since I began fieldwork as an undergraduate student in 1967.
Usually, when invited to present a seminar, scientists talk about their latest achievements. That is, after all, a good way to inform others about our work. Often, however, our goals are more subtle: we also intend to impress the audience, our peers, with our prowess. This professional exposure is important, and promoting one's work in hope that it will be useful to (and, ultimately, cited by) others is a stimulus to scientific advance. And if our presentations yield additional benefits, such as job offers or a more enthusiastic review of a proposal for funds, so much the better.
To present problems at a seminar is unorthodox, because in so doing we reveal our weaknesses. Often in technical seminars, members of the audience take great delight in publicly pointing out deficiencies or inconsistencies in the presenter's research, exposing weak points in methodology or logic. Nevertheless, I did just that: I confessed my lack of knowledge on two peripheral but bothersome subjects, thinking maybe my talk would spark some interest or even lead to ideas for technological applications I had never considered. This was, after all, Los Alamos National Laboratory--the place where the atomic bomb was born, and the place that had continued to bring in brilliant scientists to fight the Cold War.
I stood in the auditorium before a gathering of about a hundred scientists and technicians, hoping that the combination of "big dinosaur," "local excavation," and "looking for technological ideas" would pique their interest. I posed two specific questions. First, is it possible to see traces of soft tissue that may be preserved next to the bones, perhaps as unseen chemical signatures of the outline and position of muscles or stomach?
Occasionally soft parts of vertebrate animals are preserved with bones: fossil tendons are common; wing membranes of pterosaurs and bats have been recognized with some skeletons; feathers have been found with fossil birds. In a few cases, contents of the body cavities have been preserved. As a graduate student I had published a paper that described three Cretaceous fish fossils as containing probable egg masses (in a research project where I puzzled over the chemistry of preservation). And I am still intrigued by the problems of chemical alteration in fossilization.
The idea that ghost outlines might be preserved with a skeleton drew considerable interest at the seminar. I could feel the abrupt change of attention in the audience. No longer were they polite and dutifully courteous. Here was a problem these scientists could relate to, a challenge that might bridge the gap between my Victorian-style approach to fossils and their world of high technology. I sensed their tension, and my confidence grew. I went into more detail than I had planned: I gave an overview of the problems of preservation chemistry. How, in fact, do bones become fossilized?
My allotted time was short, however. I moved on to my second question: Is there any way to "see" into the ground before excavating a skeleton? Is there some technology that can give me a kind of X-ray vision so I can know whether and where to dig?
I told them why I was interested; I told them about the gigantic tail vertebrae that had been discovered an hour's drive of Albuquerque. I told them why the articulated character of the bones made them specially valuable--and seductive. And I told them of my hopes of following the tail forward, into the mesa with its ten-foot cap of sandstone.
I knew it was a ludicrous wish: to see a skeleton beneath the ground before striking the first rock with a pick-axe. With the frustrations of a century of paleontologists before me, I conveyed to my audience the difficulties we faced in excavating this exceptionally large sauropod: a ten-foot wall of sandstone to move, wilderness advocates demanding minimum disturbance and no mechanized equipment, and the likelihood of needing to race to complete the excavation before the area becomes a formally designated wilderness. If we could see the buried bones in the ground before excavating, we could dramatically improve our efficiency and minimize the disturbance.
At the conclusion of my fifteen-minute presentation, I asked for help. I expected one or two takers. Instead, I was swamped with volunteers and ideas. They overwhelmed me with enthusiasm. That seminar proved to be the most productive quarter-hour talk in my career.
Los Alamos scientists took up the challenge. On a field trip to the site, Nate Bower, a contract researcher from Colorado College, found a bone chip that he took back to his lab for chemical analysis--the results would prove surprising. Carrie Neeper, a microbiologist from the city of Los Alamos (but not at that time employed by the lab), became one of the local coordinators for volunteers and information sharing. Later, geologist Kim Manley, also from the town of Los Alamos, took an interest in gastroliths.
News of my talk and my challenging questions went beyond Los Alamos. Roland Hagan, an electronics technician at Los Alamos, enlisted the collaboration of Cliff Kinnebrew and other scientists from Sandia National Laboratory in Albuquerque to join with Los Alamos in their radar experiments. Later, Roland invited scientists led by Alan Witten from Oak Ridge National Laboratory to try their hand with technology still under development for locating buried hazardous wastes and other classified applications. By 1987 the friendly rivalry between the scientists from these three national laboratories seemed to be producing tangible ideas for assisting the excavation of Sam.
The Seismosaurus excavation had become THE SEISMOSAURUS PROJECT, a multifaceted experiment involving not just traditional paleontology, but also chemistry, physics, engineering, electronics, and a little bit of magic--magical science and magical friendships.
On one visit to the site by Los Alamos scientists, chemist Shaun Levy took hold of the fact that dinosaur bones are often preserved with relatively high concentrations of uranium. An earlier analysis at Los Alamos established that Sam's bones contain a small amount of uranium, too. The origin of this uranium is somehow related to percolation of ground water long after burial, but the actual process of deposition and concentration is problematic. Because some uranium-containing minerals fluoresce under ultraviolet light, we wondered whether Sam's bones had adequate concentrations of uranium and the right minerals to fluoresce.
We collected a fist-size fragment of bone on-site, and I accompanied the group back to Los Alamos to witness this test of fluorescence. We needed only an ultraviolet lamp and a place dark enough to conduct the experiment. Someone suggested the men's room. It's only big enough for two people, or uncomfortably, maybe three, but it can be made absolutely dark. So, several of us crowded in, turned on the ultraviolet lamp, and turned off the lights.
The fossil bone glowed. Whether the fluorescence came from the uranium was still uncertain, but at least we had discovered an unusual and potentially important property of Sam's bones, and perhaps many fossil bones.
Our discovery that dinosaur bones can fluoresce, we learned later, had been made by rock hounds long ago. This fact was well known by amateur collectors, a spin-off from the widespread use of ultraviolet lights to prospect for certain minerals in mines and caves. This fluorescence was new to us, however, and we soon learned that the glow comes not from uranium minerals in the fossil bone (uranium is there in significant concentrations, to be sure, but not in minerals that fluoresce), but instead from the natural fluorescence of the hydroxyapatite, a crystalline mineral found in all living bone--and, incidentally, probably all fossil bone in its original or nearly original state.
The discovery of fluorescence in Sam's bones suggested an immediate practical application. Because the bones were buff-colored and difficult to distinguish from surrounding rock, perhaps we could use ultraviolet light to prospect for more bone. So on a dark, moonless night our team of prospectors waited until nearly midnight to try so-called black lights we brought from Los Alamos. In three small groups, armed with flashlights to guide us to the broken cliff face and black lights to search for bones, we spread out over the site. One group searched where bones had already been excavated. Another searched where bone fragments were known to be exposed--and which we had specifically marked for testing that day. The third group searched on the face of the cliff.
The experiment was wildly successful. We found bone everywhere--most of it in small fragments that had weathered out and disintegrated over the past thousand years. Some of the bone we hadn't seen before, but none of the discoveries actually led to new intact bones in the mesa. Nevertheless, I was delighted. The night's work had made me confident that the skeleton had not been exposed and eroded away with boulders and rocks and pebbles in the cascade of rubble on the hundred-foot slope beneath the site. Rather, if more bones did accompany the eight tail vertebrae, then they were still safely preserved within the mesa--albeit beneath a cap of rock that would make life difficult for the excavation crew.
Another spin-off from this discovery of fluorescence helped us improve our laboratory preparation of several of Sam's vertebrae. One vertebra from the tail was encased in rock that was especially hard. Removal of that rock would be difficult; the work would be slow and tedious, progressing by only a few square centimeters a day. The problems were compounded by the intricate folds and projections of the bone, which were tough to follow without damaging the bone's surface. More frustrating, however, was a peculiar condition of preservation that we found on many of the upper surfaces of the bone throughout the skeleton: the sandstone rock actually penetrated the fabric of the bone, through an interval of several millimeters, destroying the naturally sharp contact between bone and rock that is common to most fossil bone. To make matters worse, the rock and the bone were identical in color, and almost identical in texture. We found that a technician could easily dig right through the bone structure and never realize it.
 |
| A partially prepared Seismosaurus caudal vertebra. The sandstone in which this bone was encased was so perfectly matched in color and texture that distinguishing bone under ordinary lighting conditions (such as photographed here) was almost impossible. |
 |
| The same vertebra under low-intensity ultraviolet light and reduced natural lighting. |
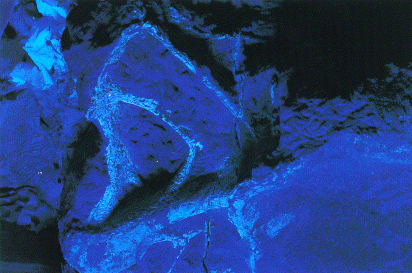 |
| Close-up of ultraviolet image. The brilliant fluorescing purple is bone and the nonfluorescing material is sandstone. Wilson Bechtel completed the meticulous preparation of this vertebra in a makeshift box that was illuminated only by ultraviolet light. This visual enhancement doubled or even tripled his efficiency. Courtesy of Wilson Bechtel. |
To solve that problem we improvised an experiment using ultraviolet light to see whether we might readily distinguish bone from rock in the laboratory, where the majority of bone extrication must be done. In the makeshift black box, which blocked out all ambient light and allowed only ultraviolet light from an overhead fixture, the bone glowed a brilliant blue and orange tint. The surrounding sandstone remained dark and unreflective. With the aid of the black light in otherwise total darkness, Wilson Bechtel prepared that vertebra with delicate accuracy and efficiency; the rate of exposing the bones improved to as much as a square inch a day, sometimes even more. We were elated.
These modest beginnings eventually led to a major research investigation of the chemistry of fossil bone preservation, including the vexing problem of why uranium accumulates in fossil bone. A preliminary chemical analysis of the fossil bone fragment I had casually given to Nate Bower was surprising: a dozen major elements in the composition of Sam's bones were of the same concentrations as that in samples of modern bone. The match was, in fact, almost identical. The conclusion was inescapable: the dinosaur bones could not have been replaced by secondary minerals. These were not stone bones; these were real bones. A large portion of what remained of Sam must be original material.
Research concerning preservation chemistry began with Nate Bower's report, which stimulated Los Alamos to get further involved. They, in turn, recruited colleagues to take up the challenge I had presented in seminar several months before--now modified to address the entire question of geological processes that lead to bone preservation. I remember having been taught about the mysterious process of "molecule-by-molecule replacement" believed to occur in fossilization. But when pressed, I could only confess confusion.
From these rather casual beginnings George, Roland, their colleagues, and I began to identify specific problems that required carefully controlled experiments. We recruited other scientists, asked for advice, held rump session seminars, and prodded colleagues well beyond the bounds of New Mexico to lend a hand. But research into chemical preservation of fossils was not the only spin-off of my Los Alamos seminar.
A century ago during the golden age of dinosaur excavations, and even thirty years ago, the principal or even sole objective in dinosaur excavations was the procurement of exhibit specimens. Today, however, the sedimentary context holds equal importance to the bones. Paleontologists now give attention to habitat interpretation (largely for improving our understanding of behavior). And we try to accurately correlate the stratigraphy of a new site with previous excavations (as precise data can be used to understand regional or even global biotic changes such as migration and extinction).
Whereas our objectives are different today, the techniques of excavation have changed little in the past century. We still use hammer and chisel, pickax and shovel--all powered by muscle and cooled by sweat. Sometimes now we do use jackhammers, driven by generators and compressors. Plaster-and-burlap bandages, often reinforced with lumber and steel, have replaced rice paper and flour paste for stabilizing bones. But, overall, we use the same procedures for finding and digging bones.
Every field paleontologist has a sad tale of discovering a portion of skeleton and launching an excavation only to find that the specimen was at the end of its erosional history, not the beginning. The only bones there were the ones exposed; the skeleton did not "continue into the hill." The immense disappointment may, however, be forgotten in the quest for another skeleton, but we all want to use our time and budgets efficiently.
Our big problem, our perpetual frustration is that we cannot see into the ground. We use experience and intuition to predict more bones below or beyond the ones that lured us there in the first place. But the only way to test such predictions is to dig. No amount of wishing or dreaming can determine how much of the skeleton is still buried, locked in the rocks beneath our feet.
No rock is too hard, no mesa too tall, to deter us once we declare a skeleton important enough to excavate. Sam's bones, encased in some of the hardest rock I have ever experienced in an excavation, and buried by ten feet of sandstone cap rock, presented a real challenge. Because Sam's tail bones were articulated and the skeleton was lying in a position that indicated curvature going into the hillside (rather than out of the hill--that is, eroded away and disintegrated into fragments in the rubble at the foot of the mesa), I was convinced that excavation was in order.
Even if Sam were not new to science, the skeleton deserved complete excavation just because the bones were connected, a rare situation. Surprisingly few dinosaurs, even the famous ones, are known from reasonably complete skeletons. But here was a skeleton only an hour's drive from our museum, belonging to a dinosaur new to the state, and in an exquisite state of preservation. We launched the excavation, with outside funding assistance, and with quiet resolve to find every bone and to carry the work to a reasonable completion.
My determination was edged with apprehension as we laid out the excavation plans. Sam's skeleton might continue for fifty or sixty feet into the mesa, and we couldn't predict with any real confidence its orientation--beyond the rather safe conclusion that it indeed went in. The skeleton might be disarticulated and its bones scattered. Predicting where to dig would require me to call on all my experience. But there would also be a lot of guesswork, and perhaps uncalled-for conviction--and (I hoped) a good measure of luck.
With measuring tapes we determined the projected depth of the bones beneath the cap rock: ten feet at least and perhaps as deep as fourteen feet (depending on the orientation or trend of the skeleton). On top of the mesa, we laid out a rectangular area that I predicted should contain the skeleton. The quarry site would penetrate sixty feet into the mesa, leaving a gap thirty feet wide.
If only we could see into the ground and know exactly where to dig before commencing the excavation, we could calculate how much rock to move, the limits of the quarry, the projected costs and duration of the work. This is a paleontologist's dream.
But "seeing" into the ground had become reality for fieldworkers in other professions. Archaeologists had pioneered stunning field applications of technology in the past two decades in their searches for buried pyramids and pueblos. Geologists had developed sophisticated techniques to follow buried river beds in the Sahara, or to detect fault lines invisible to the unaided eye. Why not try to find buried dinosaur bones? I knew the principal difference in my plea was a matter of scale: dinosaur bones, even the largest bones of a skeleton, were two or three orders of magnitude smaller than the underground targets archaeologists and geologists had set their sights on. At best, we might expect a cross-sectional diameter of a meter for the largest bones; most would be smaller.
Sam, however, proved to be an ideal dinosaur for a set of experiments conducted by several teams of scientists from Los Alamos National Laboratory, Sandia National Laboratory, and Oak Ridge National Laboratory in Tennessee. The skeleton was buried beneath a relatively uniform sandstone, without intervening layers of other kinds of rock; it promised to be articulated and relatively easy to follow into the mesa with excavation; and the bones were among the largest known. For a smaller dinosaur, at a different site, we would surely have encountered many more variables and we would have demanded even higher resolution than needed to detect Sam's bones. These were fortuitous advantages.
In effect, I was asking these scientists as volunteers on their off-duty time from their national laboratories not only for a novel application of their expertise and equipment. I was also asking them to push the resolution of their technologies and their interpretations to ridiculously small dimensions. But the teams took up the challenge.