 8. Mysteries of Fossilization |
 8. Mysteries of Fossilization |
Lot's wife, disobeying a warning, turned to look back on Sodom as she fled the evil city; for her disobedience, she was turned into a pillar of salt. In a moment, this woman changed from a living, breathing, moving human being into an inanimate rock. Instant fossilization.
For more than a century, paleontologists have explained fossilization in a sleight-of-hand manner. The explanation has stood, despite its weakness, because few professionals have concerned themselves with how fossils came to be fossils. The conventional wisdom that emerged with this laxity has allowed us to ignore what we do not understand.
Today most textbooks in paleontology delve into the subject of preservation at least superficially. The processes involved in preservation seem to be better understood for fossil plants and invertebrates than for vertebrates. Because the chemistry differs among these groups of organisms (shells do not have the same composition as bone, for example), the processes of fossilization must follow different paths. The subject of preservation chemistry of fossil bone is woefully neglected in textbooks and specialized journals alike.
However, a few paleontologists (taphonomists) have addressed the issue of fossilization directly, especially its early stages. These connoisseurs of fossil forensics have dedicated considerable effort to understanding what happens to an organism from the time it dies until long after it is buried as a carcass or a skeleton. The period from the death of an organism until it is discovered as a fossil, thousands and millions of years after it lived, encompasses its taphonomic history . Taphonomists generally emphasize the postmortem, preburial history in their studies, and only superficially consider postburial history, which is remote and largely chemical.
Consider the early taphonomic history of Sam, as described in the previous chapter. This sequence of events culminated in burial, itself a rare event, since most carcasses disintegrate and return to the soil or they are incorporated into the local food chain. This preburial history may take a few days or weeks, from the moment of death till burial, or even many months and perhaps a year or more. This is the usual scope of interest to taphonomists.
The remainder of the fossilization process and subsequent exposure through erosion or excavation is the postburial history. If a mammoth carcass and skeleton laid on the ground for ten years before the bones were fully buried, and it is discovered 10,000 years later, the period of burial is 9,990/10,000--or 99.9 percent of its taphonomic history. If it took ten years for Sam's bones to be fully buried, and because Sam lived roughly 150,000,000 years ago, the postburial years constitute 99.999995 percent of the taphonomic history.
Probably most organisms that are successfully buried (avoiding full recycling into the ecosystem) never become fossilized. In-the-ground changes destroy most organic materials, leaving nothing but chemical traces, if anything, as evidence of the past existence of organic remains in the rock. Only rarely do organisms or parts of organisms fossilize, which is really just another way of saying their disintegration is interrupted. We know almost nothing about the postburial history that leads to fossilization. We depend on untested generalizations to explain how this process of petrification proceeds. Such generalizations usually assert that preservation of fossil bone is a process of "molecule-by-molecule replacement"--a convenient, but ultimately vacuous, explanation that originated in the scientific literature over a century ago. In truth, the changes that occur after burial are complicated and not so easily dismissed.
For bones at least, the process probably never involves molecule-by-molecule replacement. In a broad sense, these are really questions of preservation chemistry: How are the fossil bones of a dinosaur different today from what they were 150 million years ago? Are they replaced? What, after all, does "replacement" mean?
In the mineral sense replacement is "the development in an old mineral of a new one that differs from it wholly or partly in chemical composition" (Funk and Wagnalls College Dictionary ). With respect to shells of mollusks, for example, this means that one crystal form of calcium carbonate (CaC03) may replace another crystal form of the same chemical composition: calcite may replace the original aragonite in the shell of a particular clam. Or the chemical composition itself might change: pyrite (FeS2) might replace calcite (CaC03), although the superficial form of the original shell is maintained. Is that how bone becomes fossilized, or "petrified"? Is the process a change from bone to stone, as we imply when we use the term replacement ?
Most conspicuous is the form or shape of a fossil. Its shape resembles a fern frond that is compressed; therefore it must be a fern fossil. Or, its shape resembles the form of a femur; therefore it must be a femur. Often the shape is definitive, and we need go no further in our examination of the object to determine its identity. The form, or shape, of a fossil is thus its most important property. This feature alone becomes the basis for identifying a fossil, the attribute we examine when we have to make a decision whether to keep it (deposit it in a repository) or discard it.
But the two dimensional and three-dimensional traits are sometimes obscure until we get a "feel" for what we're looking for, as is often the case in the discovery of dinosaur track sites. Shapes can also be misinterpreted easily, leading to embarrassing mistakes. Form alone may not be sufficient to prove an object is even a fossil--much less a particular kind of fossil. We're often shown pseudofossils, or "foolers": they might have the correct form (usually a long stretch of the imagination), but they lack texture and structure, and on full examination show inappropriate chemical composition or association with the wrong kind of rock. They have form but not substance. The process of fossilization thus must preserve more than form.
Sometimes paleontologists, like medical doctors, want to see the internal structure of a bone--the microscopic details of its anatomy. A medical doctor examines under magnification a tissue sample of bone (a biopsy), perhaps prepared with a special cutting instrument called a microtome. Or, if there is grave concern, the medical specialist might want to examine the structure of a bone (or any other tissue) under much higher magnification, using electron microscopes for resolution smaller than the wavelengths of light used by an optical microscope. At all levels of magnification, the doctor can analyze the internal structure of the bone; cells are evident, and at high magnification internal anatomy of the cells is discernible. Paleontologists can perform the same task, using samples cut not by a microtome but by precision saws that can cut a fossil bone slice to a thickness of twenty microns--so thin that light can be transmitted through it. This is called a "thin section" of a rock or fossil. Thin sections are used in all aspects of geology for microscopic examination of composition, texture, and geological history. They are as fundamental to geology as tissue sections are to medicine.
Thin sections of fossil bone, examined under a microscope at magnifications of 10X or 100X, reveal incredible detail: individual bone cells (more properly called lacunae), often as finely preserved as in a tissue section of modern bone. The boundaries of the bone cells are crisply defined, the edges as sharp as when the animal was alive. This phenomenal preservation may be indiscernible to the naked eye because of the in-filling of secondary minerals into the pore spaces of a fossil bone. Such minerals grew there long after the animal died, occupying the spaces--ranging in size from the internal marrow cavities to the microscopic lacunae--where fats and fluids and other tissues were contained in life. Often (but not always) the pore-filling mineral is quartz (SiO2), and that is what makes the fossil bone heavy, and therefore "petrified." The original cells are still intact, however--unchanged, not replaced --even though the pore spaces are full.
Besides the filling of void spaces, which makes the bone heavier (denser) than an ordinary dried-out bone from a modern animal, fossil bone differs in the way it breaks. "Green bone" breaks in curved fractures. Fossil bone, on the other hand, breaks in rectangular patterns, and the breaks feel brittle, like the break in a piece of hard candy (for example, a large candy cane). This property of fossil bone arises from the loss of protein in its structure. For the rigid portions of living bone are actually a composite of the crystalline mineral hydroxyapatite and several hundred kinds of proteins. Proteins, especially fibrous collagen, impart resiliency to bones, and allow them to bend and flex when stressed. Since these proteins decay after burial, fossil bones lose the resiliency of living bone. They break all too easily. The natural inclination of a novice to test a fossil for its breaking strength results easily in breaks, at the very point of stress where the tester tries to break it. The test, producing brittle, rectangular fractures, naturally leads to the intuitive conclusion that the bone is indeed fossilized, or "petrified."
Thus, fossil bone generally has two essential properties that make it seem different from fresh, unfossilized bone: it is heavier because of the minerals that fill the spaces previously occupied by organic fluids, and it is brittle because the collagen proteins are destroyed. Most fossil bone also loses the creamy-white or buff color typical of living bone, changing usually to a darker color and often very dark shades of brown or black. The color changes (owing to in-filling of dark minerals) may be quite variable, even within a particular excavation site.
Dinosaur bones are almost always dark, usually close to black. Sam's bones, however, are close to white. Shades of light gray, light yellow, and light brown represent the range of Sam's bones recovered at the excavation site. Sam's skeleton, and several others in the Ojito Wilderness Study Area, are the only light-colored dinosaur bones from the widespread Morrison Formation I have ever heard of or seen.
Until now, I have simply described fossil bones, particularly dinosaur bones, without critically analyzing the process by which they change from living bone to fossil bone. The idea that fossil bone is the product of "replacement" always puzzled me, for one reason: the superb quality and detail of preservation. Fundamentally, there are no qualitative differences between the solid components of modern bone (say, a chicken bone left over from a picnic) and what is now found in those exact same places in fossil bone. Only the pore-filling minerals make the two seem different. If replacement is the process that produces fossil bone from living bone, then all anatomical details at the microscopic level should be lost or blurred. Indeed, the only feature remaining unchanged should be the gross overall anatomy.
Why do I make that argument? Because in mineralogical replacement, for example, of the mineral hydroxyapatite by quartz, one crystal substitutes for another (or several). Because of their crystal growth (which mineralogists call the crystal habit), no two minerals in crystal form occupy the same three-dimensional space. For two minerals as different as hydroxyapatite (the principal phosphate mineral in bone) and quartz (the most common secondary mineral found in fossil bone), the spatial relationships are markedly different. Now, at the level of examination in hand specimens, this difference should not matter: the surface texture and form of the fossil bone should remain essentially unchanged, even if the bone has been entirely replaced and is all quartz (at this extreme, the bone would appear glassy and smooth, a rare condition).
However, at microscopic levels of examination, if replacement is responsible for preservation of fossil bone, the differences in three-dimensional properties of the crystals should materially affect the quality of detail. The cells (technically the lacunae) should be destroyed, the evidence of their past existence obliterated or substantially altered. In essence, this destruction of detail at the cellular level would be an obliteration of the sharp boundaries between lacunae, and the boundaries between lacunae and pore spaces. Yet all fossil bone I have ever examined under a microscope shows no such loss of detail at the cellular level. The microscopic anatomy of fossil bone is spectacularly and crisply preserved. Parallels between modern bone and fossil bone are astonishing, even for dinosaur bones 225 million years old. Only an expert can distinguish fossil bone from modern bone at the microscopic level, and then the only clear distinction is color.
 |
| Thin section of leg bone from Coelophysis. This bone from a small carnivorous dinosaur, excavated at Ghost Ranch, New Mexico, is roughly 220 million years old (Chinle Formation; Triassic). The thin section was cut parallel to the haversian canals, which are now filled with clay minerals. The canals appear dark in this photograph, which was taken under polarized light. The brightly colored material is bone, mainly the mineral hydroxyapatite, largely unaltered. |
Owing to a seminar I gave at Los Alamos National Laboratory (described in chapter 3), chemists took up the challenge. George Matlack, Dale Spall, and Roland Hagan from the Lab, and Hilde Schwartz, my colleague from Dixon, New Mexico, launched an attack on the problem of preservation chemistry. Actually, this inquiry began somewhat later when Roland received a chemical analysis of a fragment of Sam's bones from Nate Bower, a chemist at Colorado College.
Nate's results were puzzling: for a series of major elements, the concentration in fossil bone almost perfectly matched the concentration in a sample of modern bone. Only silicon (in quartz, SiO2) was different: high in the fossil and low in the modern bone. The similarities were astonishing. Maybe Sam's bones had not been petrified, someone suggested. No, I countered, these bones aren't any different, except for color, from thousands of fossil bones I had seen in my twenty years as a paleontologist. Sam's bones are not unique, I asserted; they instead represent typical dinosaur preservation.
But test results cannot be ignored. Testing is, after all, what separates science from speculation. If replacement were to account for fossilization, we reasoned, then the elemental comparisons between living and fossil bone should be substantially different. Nate's analysis planted the first real seeds of doubt.
Dave Mann, a Los Alamos specialist in making thin sections of rock samples (he had been one of the first to make thin sections of moon rocks), prepared thin sections of scraps of Sam's bone for us to examine. Like other dinosaur bones, the microscopic details were sharp: cellular structure was crisp, boundaries were intact, and the pore spaces were filled largely with crystalline quartz as in typical fossil bone. The high concentration of silicon in Nate's analysis was consistent with what we saw under the microscope, but how to explain the other elements? From two disciplines, analytical chemistry and mineralogy, we had complementary information. But from neither of these preliminary studies could we identify the mineralogical composition of the bone--or what it was that had the form of bone, as distinguished from the quartz infillings. Was the correspondence only a coincidence? Or, was the fossil bone real, its form and substance unchanged from its original composition?
 |
| Dave Mann and his on-site coring device. The device is a modified drill press. The hollow coring bit was lubricated by distilled water to avoid contamination. |
 |
| Bone, in situ. This hint of bone was exposed during coring. The whole piece was later excavated and identified as caudal vertebra no. 7. |
To produce fresh samples of bone at the site, Dave built an ingenious, simple coring device from a drill press and spare parts in his laboratory. Lubricated by distilled water and powered by a generator at the excavation site, the coring apparatus cut into the rock like a tubular cookie cutter. Dave and several assistants drilled three solid cores, each about two inches in diameter, from one of Sam's bones that had been exposed but not treated with preservatives or hardeners. We had only exposed a small part of the bone, awaiting sufficient funding to commence a large-scale excavation, and we weren't positive which bone in the body it would be. Later we identified it as the seventh vertebra of the tail as counted from the hips rearward. At the time of drilling we did not know how thick this exposed bone would be (I had correctly guessed it was a caudal vertebra) or whether we would drill through a thick and dense section of the bone. However, we decided to take the chance that we would get good sections in the cores, rather than expose the bone further and risk altering its in situ chemistry. The distilled water was for ensuring purity in the sampling: we did not want to introduce new chemicals to the bone and surrounding rock that might contaminate the cores and give false readings in our analyses.
These three cores became the samples for the first detailed chemical analyses of Sam's bones. Lab personnel treated the bones as though they were a precious metal. The cores were locked up in safes, paperwork was attached to every piece as though tracking nuclear material, and everyone handled the bone as though it were a newborn baby. In one meeting at the Lab, at least two dozen personnel showed up for the unwrapping of the first cores--an event akin to unveiling an artist's latest work.
The three original cores were all about a foot long. As they were crumbly, they were retained in foil jackets. Unfortunately, they contained only thin pieces of bone separated by sand that was only partially cemented. Clearly, we had not taken our samples from the main body of the vertebra, but from the edges where thin laminae support the external struts for muscle attachment. We confirmed this conclusion later, with excavation of the bone. In the cores, the thickest bone was less than a half inch--sufficient for analysis, but we had hoped for more. We kept the sand and sandstone intact so that we could analyze it too for its mineralogy and chemistry, as it was probable that the bone and its surrounding matrix were in a condition of dynamic equilibrium. Some of the chemical content of the bone would thus be leached into the sand, and some of the chemical content of the sand leached into the bone.
The dynamic equilibrium would be a condition of chemical adjustment corresponding to the temperature, pressure, and groundwater chemistry present near the time of exposure. Earlier in the burial history when Sam's bones were under considerable pressure beneath hundreds and perhaps well over a thousand feet of sediment and even deeply submerged beneath the waters of the Cretaceous sea, temperature and chemical conditions would have varied. The mineralogical and chemical properties of the dynamic equilibrium of bone and rock matrix would thus have varied too. Indeed, this is the guiding principal in understanding all chemical changes of rocks and minerals in the earth, and it applies equally to buried fossils: neither the surrounding rocks nor the fossils are inert. They are mutually affected by conditions of burial, their components interacting in chemical reactions that should be predictable. Assuming dynamic equilibrium as the guiding principal, the implied quest to identify the predictable chemical properties became our goal.
A less inquisitive group of scientists might have found Nate Bower's results sufficient to claim that the mineralogical part of Sam's bone (as opposed to the organic components such as proteins and fats) was essentially unchanged from its original chemistry. But we wanted to probe more deeply. For example, could the elemental chemistry be the same, but the minerals entirely different? And did the bone contain organic remains as well?
Understanding the chemistry of fossil bone is no easy problem. Living bone contains a multitude of chemicals in both organic form, such as fats and proteins, and inorganic forms such as the crystal structure of hydroxyapatite. In life, bone is continually growing and changing through the course of an individual's existence. Even after "growth" has ceased in adult humans, bone is alive, responding to stresses and changes just as other parts of the body change. Fossil bone preserves much of the structure or form of the original living bone, but what about its composition? Surely the organic portions on the whole are unstable, but some or parts of some may be durable enough to survive millions of years of burial. Amino acids have been recognized in invertebrate fossils from the Paleozoic Era. Is it possible that amino acids and proteins might be found in dinosaur bone, especially in bone so well preserved as Sam's? We faced two tasks: identify the mineralogical component of the fossil bone, and try to extract, even in small concentrations, the organic parts.
The mineralogical component of fossil bone is principally
hydroxyapatite. It is a relatively simple crystal laid down The proportion of fluorine is important. Fluorine may be incorporated
into living bone in small concentrations, especially into the dentin of
teeth, which is a specialized form of bone. The addition of fluorine
strengthens bone, making it harder and denser, which imparts resistance
to bacterial decay in teeth. With sufficient fluorine in its composition,
the mineral name changes to fluorapatite, a variety of hydroxyapatite
with essentially the same crystalline properties as its precursor. In
isolation, a single crystal of hydroxyapatite or fluorapatite resembles a
prism with six sides, expressed technically as a hexagonal crystal habit.
As bone grows, the mineral crystals grow initially in isolation, in a
matrix of collagen fibers. The hydroxyapatite provides strength, the
collagen proteins resilience and flexibility. Together, they make a
remarkable material, capable of withstanding considerable compression,
torsion, and stress, and forming the anchor for muscle action that
propels the limbs and moves the various parts of the skeleton.
The highly organized microscopic architecture of bone develops
according to direction of growth, stress fields in the bone, and position
with respect to the internal anatomy of the bone. Much of the growth is
under control of electrical stimuli generated from stress on and in the
bone. The crystals of hydroxyapatite grow in an alignment parallel to the
blood vessels contained within the bone, the haversian canals. The
regular spacing and alignment of these crystals impart the strength of
bone. Insufficient calcium in the diet impedes growth and maintenance of the hydroxyapatite crystals and may cause structural
deformity, such rickets in children, because the bones cannot properly
endure the weight of the body. They respond by bending and changing much
as a pole will bend if overloaded.
In living bone, the orientation of the crystal axis of the
hydroxyapatite is controlled by the direction of growth of the crystal.
Generally, the orientation of the prismatic crystal is normal (that is,
perpendicular) to the stress loading and parallel to the growth of the
circulatory channels that carry blood and other fluids that bathe the
internal tissues. Like the hydroxyapatite, the haversian canals grow
normal to the stress loading; the bone that bounds the canals contains
the regularly spaced hydroxyapatite crystals. Thus, the crystals and the
orientation of the canals are parallel in living bone.
The canals are part of the form of the bone. They can be observed in
microscopic detail in both living and fossil bone. They almost always
appear to be unaltered in fossil bone, except their cavities may be
filled with secondary crystalline minerals such as quartz. The
hydroxyapatite crystals, on the other hand, are not part of the overall
form of the bone, since they are exceedingly small and not readily
visible even at high magnification under an optical microscope. According
to expectations arising from study of crystalline materials in the
geologic discipline called metamorphic petrology, the crystalline
orientation of the hydroxyapatite should be altered with burial and
compression in response to the new conditions; the major axis of
orientation should be normal to the direction of compression during
burial--which is likely to be very different from the direction of
compression during growth. This is what we predicted for the
hydroxyapatite in Sam's bones.
What about the collagen? The matrix in which hydroxyapatite crystals
grow is a fibrous network of protein, mainly the variety of proteins
collectively called collagen. This is the organic part of the structural
components of bone. Collagens are proteins, complex molecules whose
structure depends on chains of carbon in complicated linkages. These and
other proteins are large organic molecules, thousands of times larger
than the hydroxyapatite crystals (inorganic compounds with much simpler
construction) which form in the protein matrix and give
the bone its strength. Amino acids are simpler organic molecules that are
collectively and systematically incorporated into proteins as building
blocks. Some amino acids are stable over long intervals of geologic time,
presumably preserved as remnants of the original protein. If amino acids
could be recovered from invertebrate fossils of the Paleozoic Era, three
or four times older than the dinosaur bones in the Ojito Wilderness Study
Area, then we might be able to isolate amino acids and maybe even
proteins or protein fragments from Sam's bones.
Because we needed more samples than the three we took on site with
Dave Mann's coring rig, we decided later in the course of the experiments
to take cores from one of the bones of the pelvis, the ischium. The
ischium is one of the densest bones in a dinosaur's body (that is, it is
less riddled with haversian channels and the bone is essentially solid),
and it would provide excellent samples for analysis. We had excavated the
ischium without application of hardeners or chemicals, thereby ensuring
that we had not contaminated the bone unnecessarily.
By 1989 the multidisciplinary research projects on these bones were at
a peak of intensity, and I traveled frequently to Los Alamos as a
consultant to the Chemistry and Laser Sciences division. Many of the
results led to one central conclusion: that we had original--truly
original--bone, virtually unchanged except for loss of
some material (especially organic molecules) and only moderate
modification of what was left (for example, the enrichment of fluorine in
the hydroxyapatite). Los Alamos agreed to host a workshop for
participants and anyone else with an interest in the subject of
preservation chemistry. In March of 1989 we convened the Bone Chemistry
Workshop, with three days of informal oral presentations by more than
thirty participants, including scientists from beyond the confines of Los
Alamos. The evidence from many angles was consistent: there was no
"molecule-by-molecule replacement" in the fossils of Sam's bones.
Since then, some of these experiments have been completed, or
otherwise abandoned. Others continue, especially in organic chemistry, as
new techniques are applied for ever-more-complicated analyses. These may
take several more years to finish. Many questions about the chemistry of
preservation remain to be answered, particularly with respect to the
timing and duration of each stage in the process. Moreover, the
experiments were confined largely to Sam's bones. The burial history of
Sam's skeleton is unique, the color is unusual, and many other variations
and effects of burial are possible. We cannot, thus, globalize our
conclusions with certainty--but we do suspect that other fossil bones are
similar in the amount of original bone that remains.
In the rest of this chapter I present an interpretation of some of the
experiments, and then conclude with speculation on the process of
fossilization itself as a sequence of events. The analyses focused on two
major features of Sam's bones: the nature and orientation of the
hydroxyapatite and associated minerals, and the quest to isolate proteins
and other organic molecules.
The experiments on Sam's bones suggest that the hydroxyapatite is
largely original. Most of the original hydroxyapatite is still preserved
in the bone, albeit in slightly altered form, and most of the
hydroxyapatite crystals retain their original orientation with respect to
the haversian system of the bone. Two aspects of the hydroxyapatite
crystals are, however, changed somewhat.
First, the chemical composition is enriched in fluorine (to 6 percent
composition), changing the chemistry enough to prompt mineralogists to
identify it as fluorapatite, a variant of hydroxyapatite that could as
easily be called fluoro-hydroxyapatite. Six percent fluorine is much
higher than that found in the bones of living vertebrates, and thus we
suspect that dinosaurs did not, in the flesh, produce fluorine-rich
bones. The addition of the fluorine does not change the size or shape of
the crystals, but the fluorine seems to play an important role in the
early stages of preservation (as described later).
The other change in hydroxyapatite in Sam's bones involves the
apparent growth of new crystalline material from the original
hydroxyapatite; these new crystals grew after death from preexisting
smaller seed crystals. Sam's bones now contain two sizes of
hydroxyapatite crystals, the (presumably) smaller original crystals that
are the majority of the composition and the secondary crystals that are
less frequent but one hundred times larger. These larger crystals may
have grown at the expense of other hydroxyapatite in the bone released to
solution at pressure points in the fossil, or where fractures in the
fossil allowed increased percolation of fluids that would dissolve and
redistribute the elements at nucleation centers.
The analyses demonstrated two important surprises about the
hydroxyapatite. First, compared with modern bone, the crystallinity is
more pronounced, apparently because of the loss of protein, which in turn
may have enhanced the secondary growth of the crystals after death
(probably after burial). Second, the orientation of the hydroxyapatite
crystals (the long axis of the crystal habit) still parallels the
haversian system. This orientation should have changed with burial,
pressure, and time.
The fact that the crystals remain unchanged in their orientation has
three important implications: (1) the bone has probably not been buried
deeply or subjected to high temperatures and pressures that would drive
the reorientation; (2) the crystals are remarkably durable and not easily
altered, a conclusion that should not be surprising in light of the
remarkable strength and durability of living bone, one of the most
durable materials in the natural world; and (3) the hydroxyapatite is
mostly original (especially the smallest crystals) and unaltered even in
their crystallographic orientation.
For me, this last implication was the clincher. It was proof that we
have original composition, contradicting what some paleontologists have
implied without direct evidence. The only material change is in the
fluorine, which may have been the critical factor promoting preservation
in the first place.
On the other hand, most of the organic composition is lost; only
traces remain. But these traces theoretically can be isolated and identified. Quite unexpectedly, these remnants have
been relatively stable through disintegration of the carcass, burial,
temperature and pressure changes, and now exposure again to surface
conditions. How they are preserved remains a puzzle, but the evidence for
their existence seems convincing.
Bones contain more than four hundred different proteins, most of which
are unique to bone and not found in other tissues. Collagen is the most
abundant of the bone proteins, constituting more than half of all bone
protein material. Collagen develops in the matrix between cells during
bone growth. This protein fixes the position of nucleation of the
hydroxyapatite crystals from specialized bone cells.
Los Alamos scientists Dale Spall, Lawrence Gurley, and colleagues
isolated small quantities of protein from Sam's bone samples, which had
been carefully handled to avoid contamination. The full analysis of the
protein has not been completed, but one conclusion has been reached: the
protein is not collagen. In any case, isolation of this protein, or
proteins, from Sam's bones is the first instance of recognition of
organic molecules from a dinosaur. Lawrence R. Gurley, Joseph G. Valdez,
W. Dale Spall, Barbara F. Smith, and I published these results in 1991 in
the Journal of Protein Chemistry .
The next logical step in the analysis is amino acid sequencing. It is
the only means of positive identification of a particular protein. But it
is a laborious and time-consuming process to establish the exact order of
several thousands of amino acids; it is not clear whether this work will
be done any time soon--if ever. However, the recent developments in
genetic chemistry that use polymerase chain reaction enzymes for
sequencing base pairs in DNA hold considerable promise for applications
in paleontology.
Proteins may occur in greater concentration in other skeletons,
preserved under different conditions of burial. Clearly, more work is in
order on this general problem, if not particularly for Sam's bones.
Proteins and genetic material have been isolated and analyzed from
spectacularly preserved fossil leaves from Miocene lake beds (17 to 22
million years old) near Clarkia, Idaho. Paleontologist and popular
science writer Stephen Jay Gould summarized in 1992 the occurrence of
these leaves and their evolutionary implications. (See
Gould's article for references to technical papers on the Clarkia
fossils.)
With our discovery of noncollagen protein in a dinosaur bone comes
many implications for future research on fossil bones. The potential
applications are almost overwhelming. For example, we could begin to use
comparative biochemistry of proteins and other biological macromolecules
to test phylogenetic reconstructions of the various kinds of
vertebrates--and thus determine who is related most closely to whom. We
could better our understanding of the evolution of biological
macromolecules, such as collagen and various bone proteins. We could
learn to construct a geochemical history of a given site by calibrating
the loss of sequentially susceptible organic compounds to
pressure/temperature changes caused by burial.
And if there is protein, perhaps there is nucleic acid too. The
futuristic science underlying the book and film Jurassic
Park may not be all that far-fetched.
Out of these bits and pieces of evidence from Sam's bones, it is now
possible to propose a rather tentative sequence of events leading to
fossilization of bone in general. We can be sure of the beginning of the
process and the final result: we start with living bone in a live animal,
and we end with the fossil bone as it is discovered and removed from the
ground. But what of all the events in between?
First, we must keep in mind that not all bones that initially become
fossilized remain intact in the ground; surely, much or most bone that is
initially preserved is lost to disintegration by groundwater under the
destructive influence of high temperatures and high pressures of burial
beneath hundreds and thousands of feet of sediment. Thus, the fossil
bones we discover at the earth's surface may be only a small fraction of
the bones that were preserved initially. The survivors had the right
composition and nominally optimal conditions of burial; the sequential
disintegration remained arrested or sufficiently slowed for a long enough
period of burial to allow the bone to retain its original form and much
of its composition until it reached the surface again as a consequence of
erosion.
This history is precisely what we want to understand. At any point during burial, changes in the surrounding rocks may
accelerate the disintegration and alter the fossil to such an extent that
it is no longer recognizable: its form may be destroyed, and ultimately
all chemical traces of its existence may be lost into and beyond the host
rock.
First, the animal dies--the obvious starting point in our sequence of
events. The carcass disintegrates and is subjected to the actions of
scavengers. After an interval of time on the surface, which may range
from only minutes to years, the carcass or, if it is stripped of all its
flesh, the skeleton must be buried. Skeletons that remain on the surface
(the usual and surely the majority of instances) fully disintegrate,
their substance taken up by decomposers, scavengers, and soil-producing
organisms. Only the skeletons that are buried and encased in a matrix of
sediment or rock can become fossilized.
At some point in the process, soon after burial and probably within
the first several years, percolating groundwater bathes the skeleton.
Groundwater almost always contains fluorine in low concentrations. The
bones take up the available fluorine by incorporation into the
crystalline hydroxyapatite until they become saturated in this element.
By this elemental substitution, the hydroxyapatite becomes fluorapatite.
Indeed, this may be the single most important event leading to durability
of the fossil bone. Without fluorine, perhaps all buried bones would be
lost to further destruction. (We know that Pleistocene fossil bones
between 5,000 and 1,600,000 years old also have high fluorine in their
composition, but whether this pattern is universal has not yet been
established.) The addition of the fluorine imparts resistance to further
decay, similar to the resistance to bacterial decay that results from
fluoridation of growing teeth. The bones become denser and harder.
Simultaneously, during the first several years after death, unstable
organic molecules disintegrate. These includes fats and other lipids,
amino acids, and proteins such as collagen. They probably disintegrate at
predictable rates, each with different susceptibility to local conditions
of burial. Most of these organic molecules are destroyed early in this
process, but some apparently survive. Meanwhile, the loss of organic
molecules and internal fluids leaves open spaces within the internal
structure of the bone, mainly the conduits in the bone
where the haversian system transmitted blood and other fluids. These
voids are immediately available as tiny spaces for crystal growth.
At some point in the burial history, the voids are filled with new
crystals generated from elements carried to the bone from groundwater
seeping from the surrounding sediments or rocks. Typically, these
crystals are quartz (SiO2). Oxygen and silicon are, after all,
the two most abundant elements in the earth's crust. Silicon is
universally available in groundwater, and quartz easily precipitates
under these conditions of burial. Eventually the pore spaces are filled
with the new quartz crystals (or other minerals) and no space remains for
growth or precipitation of additional materials.
This process of in-filling may be the second critical event (the first
was the introduction of fluorine) making possible the long-term survival
of the bone. Internally, the quartz crystals entirely occupy each pore
space, preventing or at least retarding the penetration of additional
groundwater and adding structural strength to the bones. With the
addition of durable crystals filling the void spaces, the bones resist
compression, an especially important attribute when the bones are in a
compressible host rock such as clay or shale. If buried in a
noncompressible rock such as sandstone or gravel, compression is less
likely to change the bones, and their three-dimensional structure is
preserved essentially unchanged.
These two events in the fossilization history (introduction of
fluorine and filling of void spaces by mineral precipitation from
groundwater) probably make the difference between preservation or
destruction of buried bone. For even after burial, bone is subjected to a
destructive environment. At least early in the burial history, decay
organisms continue breaking down the bone by feeding on organic molecules
and disaggregating the structural framework. Introduction of fluorine
must thus occur early, too, and its effect would seem to be protection,
perhaps by increasing the bone's density (or compactness) and inhibiting
bacterial decay by making the internal structure inaccessible.
I suspect that the fluorine must be introduced almost simultaneously
with burial, and that within a few tens or hundreds of years, during
which time the bones may not be deeply buried and still
remain subject to bacterial decay, the fluorine content is as high as it
will get (approaching 6 percent in the hydroxyapatite, thus becoming
fluorapatite). Also, I propose that bone not sufficiently invested with
fluorine, for reasons of local variations in fluorine availability in the
groundwater, will not fossilize; it is destined to decompose and its
substance recycle into the sediments and solutions where no visible trace
of its existence will remain. Thus, bones that get buried successfully
will have their rate of decomposition arrested or slowed sufficiently to
retard destruction. Other bones insufficiently enriched with fluorine,
even in the same bone horizon, may not survive this early stage of
decomposition, even though they are buried like the ones that survive.
Such differential loss of bone after burial, whereby some bones (or
skeletons) survive post-burial decomposition and others are lost
entirely, may seriously affect our efforts to census populations of
extinct animals based on the abundance and density of their occurrence as
fossils. In fact, disintegration of bone might explain why some
sedimentary rocks contain no fossils. We often conclude that, because we
find no fossil bones in a given formation or a given geographic area,
fossil animals did not live there, or at least their skeletons were not
buried there. This casual assumption may not be correct: in many cases,
the differential loss of bone for want of fluorine may erase the evidence
of their existence, leading us to incorrect conclusions. Paleontologist
Robert Bakker has suggested a variation of this idea, elaborating on the
notion of differential preservation of gastroliths and skeletons. By his
argument, clusters of possible gastroliths in some geologic formations in
the American West (especially the Morrison Formation) may have survived
intact while the skeletons in which they were deposited and buried have
dissolved and all vestiges of the bones have vanished.
Several observations lend support to these conclusions. First, in my
work with Pleistocene mammals and reptiles, I have often found the bones
to be soft and chalky. This is true in some sites (but not all) from
Florida to Michigan, and from the East Coast to the deserts of the
American West. This texture probably arises from disintegration of the
bones during burial, and they would eventually be lost.
Bones older than Pleistocene are almost always hard. Evidently,
disintegration of buried bones is completed within the first 1,600,000
million years or so, and I suspect even within the first 100,000 years.
In other Pleistocene sites bone is hard and well defined, even bone that
is only a few thousand years old. A useful study would be to test for
differential concentrations of fluorine in the chalky versus the hard
Pleistocene bones. (In a different vein, it would be interesting to
determine the effect of embalming chemicals on fluorine uptake in bone;
it is possible that by embalming corpses we inhibit the incorporation of
fluorine in buried bone, and consequently hasten the loss of skeletal
material.)
Another observation that lends support to my ideas about long-term
preservation of bone is that dinosaur bones are always filled with
accessory minerals, such as quartz, that make them heavy; on the other
hand, Pleistocene bones often retain their pore spaces, with little or no
growth of accessory minerals. Teeth seem to be the most durable skeletal material. Often we find
only teeth and no bones; consequently, for some groups of vertebrates,
teeth are the principal focus of attention in classification. This is
especially true for mammals, and notably for Mesozoic mammals and
mammal-like reptiles. Fortunately, mammal evolution at least partially
followed a pattern of feeding specialization, which is reflected in the
structure of the feeding apparatus, especially teeth. Thus, teeth are
among the most desirable elements in a fossil site, and especially a site
yielding mammals. I have always been puzzled about the differential
preservation of teeth over bones. Teeth seem to be favored by the
processes of fossilization to a greater extent than bone, perhaps because
the internal structure of teeth (enamel especially, and
dentin) is already dense at the time of death and burial and therefore
resists decay more effectively than bone.
Finally, consider that fossil skeletons of adult animals are much more
common than skeletons of babies and juveniles. But this surely does not
reflect the actual population structure. Instead, it likely owes to the
fact that the rapidly growing bones of young animals are more extensively
invested with blood vessels and corresponding void spaces at death,
subjecting these bones to more rapid disintegration before and after
burial. Then, too, the bones of the youngest animals would likely be
ingested with flesh by predators and scavengers, whereas the bones of
adults might instead be picked.
These arguments (the chalky texture of some Pleistocene bones, the
strengthening added by quartz infilling, a preservation bias in favor of
teeth, and a preservation bias in favor of the denser bones of adult
dinosaurs) all fall into the general category of "differential
preservation." It may be the most difficult process to analyze because
the differentiation results in an either/or product: either the buried
bone is preserved (fossilized) or it is not. The null case cannot be
studied, with the possible exception of the chalky Pleistocene bones that
may represent fossils that would not make it beyond a few tens of
thousands of years.
Bones that survive these early stages of disintegration after burial
may still be subject to destruction and alteration from changes in
subsurface conditions. The dinosaur bones buried in the Morrison
Formation of the western United States were beneath hundreds and
thousands of feet of additional sedimentary rocks during much of the
Cretaceous Period, and many of these sediments were deposited beneath
shallow and not-so-shallow seas. Introduction of marine waters to the
underlying rocks certainly changed the conditions of fluid chemistry in
the sediments surrounding the bones. The deep burial produced increased
pressure, it elevated temperatures, and changed the nature of the fluids
in the rocks surrounding the bones; all of these changes should have
considerable effect on buried bones, although this speculation is hard to
test. Perhaps the bones are already so resistant to destruction that
these changes would have little effect, but I suspect
that many bones are lost to these changes introduced from the processes
of deep burial.
The elevation of temperatures through the geothermal gradient (an
increase of ten degrees Centigrade per thousand feet of burial), probably
affects organic molecules more than the inorganic composition of bone.
Probably all vestiges of proteins and simpler organic compounds are
destroyed by high temperatures; only bones that (like Sam's) were never
deeply buried and therefore not subjected to markedly elevated
temperatures will have residues of organic molecules. Relatively
speaking, Sam's burial site has not suffered from great changes in
pressure and temperature. Because the Ojito is in the thin edge of a deep
basin (in which burial was deep in its middle) at the margin of the
Colorado Plateau, its burial history is simple. We know from analysis of
clay minerals that the temperature of these rocks never exceeded 100°
C, a conclusion that indicates the rocks have never been subjected to
heavy loading by deep burial. In other words, the sea was never very deep
here, and there was never a great thickness of rocks overlying this site.
Until fossil bones are exposed at the surface of the earth, they are
sealed in their host rocks in a state of quasi-equilibrium. With each
physical and chemical change in the conditions of burial, the bones
change too, but the hydroxyapatite seems to be highly resistant.
Apparently, the destruction of hydroxyapatite is accomplished only with
extreme changes, whereby the surrounding rocks are subjected to high
pressures and temperatures that drive all chemicals to changes in crystal
structure and mineral composition under new conditions of equilibrium.
Provided that the bones survive these gradual changes during their
burial history, they may be freed eventually by erosion, as overlying
rocks are stripped away by the forces of water, wind, and gravity. In the
case of exposed sections of the Morrison Formation, the overlying rocks
may once have been a thousand feet thick or more (depending on location).
Stripped away by erosion stimulated by the uplift of the Colorado Plateau
and surrounding areas, the eroded sediments were then carried away by
streams and rivers.
The removal of overlying rocks brings new trials to buried bone. Changes in ground water generated by rains and
saturation zones in water tables may subject the fossil bones, now
relatively shallow, to changes in acidity and alkalinity, and introduce
elements that can precipitate as new minerals in and around the bone.
Often we find fossil bones encased in extremely hard rinds of rock, which
we collectively call concretionary matrix. This was the case for most of
Sam's bones, but not all. I suspect that generation of concretionary
materials around bones may occur relatively late in the burial history,
not long before exposure at the surface of the earth. In dinosaur bones,
this might happen during the last million years of their burial
(remember, for dinosaur bones buried for 150 million years, like Sam's,
the last million years of burial is less than 1 percent of its existence
as buried fossil bone), and the process may not equally encase all bones
in a given site or a given skeleton. For example, about a fourth of the
surface area of Sam's bones was free of concretionary sandstone, and
could be simply swept clean, or required only minor preparation to expose
the bone. But most of Sam's bones were encased in the extremely hard
concretionary layer, a rind that penetrated into the fabric of the bones
and made laboratory preparation exceedingly difficult.
Eventually, bones that have been buried for eons may be exposed at the
earth's surface. Overlying rocks stripped away by erosion, just to the
level where the bones occur, the bones may remain in position and be only
partially exhumed. This was the condition of the original eight tail
bones of Sam's skeleton that we excavated in 1985. Part of the skeleton
(the distal half of the tail) had been wholly exhumed, fragmented by
erosion, and carried away as sediment, and part of the skeleton remained
buried and unexposed; that was the part we subsequently excavated.
and surrounding areas, On reaching the surface, the bones are
intensely affected by erosion and weathering, especially the action of
freeze-thaw cycles that can reduce quality bone to fragments in a matter
of months or years, especially bones that are not case hardened in a
concretionary matrix. Once exposed, fossil bones do not last long; their
usual fate is mechanical destruction, although some are collected by
paleontologists and others may be recycled and become part of the newly
deposited sediment in the stream beds that carry away
the products of surface erosion. Forces of destruction are severe on
exposed bones, a scientific tragedy in the sense that the bones have
survived millions of years of burial only to be destroyed by the ordeal
of surface exposure. Add to these natural causes the deliberate and
accidental destruction of fossils by people, and it seems almost
miraculous that any bone should survive long enough to become a museum
specimen.
With so many situations working against preservation, we are truly
lucky to find any fossil bones at all. Each stage in the preservation
history is a kind of filter--or grim reaper. With each filter, surely no
more than 10 percent persist. Probably 1 percent is a better estimate
(but probably not as low as 0.1 percent). This latter figure would lead
to an estimate of close to one trillion individuals in a given population
of dinosaurs for a representative century.
Thus using the 10 percent figure, from a population of, say, a million
individual dinosaurs that died in a given century during the late
Jurassic, perhaps only 100,000 skeletons were buried successfully; 10,000
survived decomposition during early stages of burial; 1,000 survived deep
burial; 100 survived shallow burial; and only 10 became exposed. Of those
10, only one survived on the surface long enough to be discovered by a
paleontologist. That was Sam. One in a million.
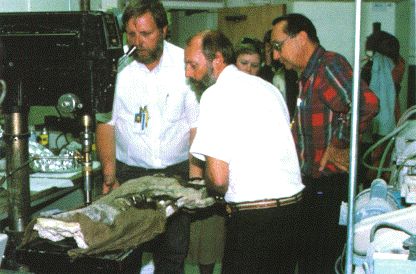
Los Alamos National Laboratory scientists. Dale Spall, Dave
Mann, and Lawrence Gurley (left to right in the foreground) take a solid
core from Sam's ischium for chemical analysis.
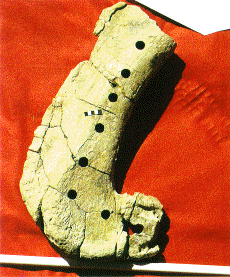
Ischium after samples extracted. The samples were extracted in
the laboratory, after removal of the rock matrix. We chose the ischium
for chemical analysis because its bone is among the densest in a dinosaur
skeleton. Having fewer voids, it is subject to less in-filling by quartz
during fossilization.
Thin section of Sam's bone (normal light). The internal
structure of the bone is sharply defined, even under the normal light
used in this photograph. Photo courtesy Las Alamos National
Laboratory.
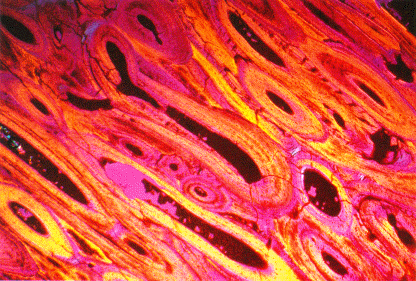
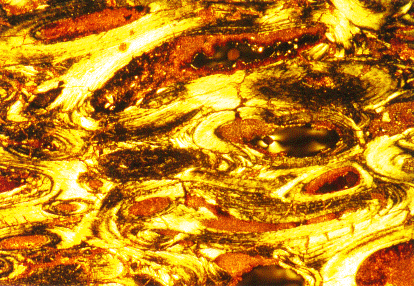
Thin section of Sam's bone (polarized light). Polarized light
enhances the definition of internal structure. The section was cut across
the haversian canals; the dark centers of the elliptical figures are the
former canals, now filled with clay minerals. The canals are surrounded
by a dense growth of bone arranged in layers that grow parallel to the
haversian system. The hydroxyapatite crystals and the shape of the bone
cells that secreted them (too small to recognize at this magnification)
are present in Sam's bones in a state almost identical to their condition
at the time of death. Photo courtesy Las Alamos National
Laboratory.