

Primary chemical structures: limestone encrustations
Plate 163




Primary chemical structures: limestone encrustations
Plate 163
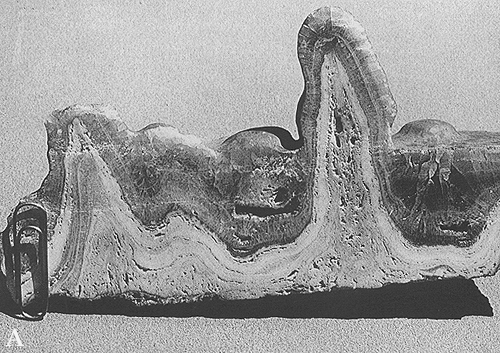
Plate 162 A and B document two details of
speleothems, as cave deposits are called. A stalactite has
been cut, and the section shows two distinct generations of chemical
precipitates, both consisting of calcium carbonate. The spongy inner zone
(dripstone) is the result of dripping from the cave ceiling; the banded
outer zone (flowstone) reflects, instead, the slow growth of crystals
from solutions flowing along the solid surface. Flowstone
deposition seals and drapes the dripstone, which implies a change in
water circulation in the karst system. All cave "formations" (the term is
used by speleologists for morphological varieties of precipitates, which
geologists would call "structures": stalactites, stalagmites, basins,
ponds, etc.) owe their origin to the release of carbon dioxide from water
percolating through the karst system. Carbonate ions are present in
solution, and the escape of CO In plate 162 B it can be more clearly seen that: 1) flowstone
bands are sets of depositional laminae, recognizable by differences in
color; 2) the laminae are made of elongated crystals, which are oriented
normally to the lamina surface; 3) the parallelism and continuity of the
laminae is remarkable; a small displacement is observable, however, along
a fracture on the right (a micro fault). Flowstone laminae are possibly
organized in seasonal rhythms (varves ) and longer-term
cycles, as indicated by minor and major color changes between individual
laminae and laminasets, respectively. The color of pure carbonate is
white; darker hues are due to variable concentrations of impurities (iron
oxides and hydroxides). The major changes in impurity content should
reflect multiannual hydrologic-climatic changes.
Calcite crystals formed the laminae by growing side by side and
disturbing each other; maximum growth then occurred perpendicularly in
the free space of the cavity, which explains their elongated shape. This
banded cave deposit is used as ornamental stone with the name of
calcareous alabaster.
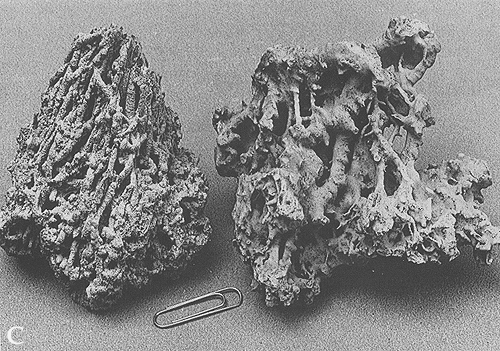
In plate 162 C, the carbonate encrusts vegetal remains.
Precipitation can be restricted to the vicinities of springs or falls, or
be more extensive and form continuos layers. Travertine
deposits, or tufa, characterized by a spongy or vuggy texture, can thus
accumulate. Their thickness can reach tens of meters; several travertine
quarries are open in central Italy. The stone is used as a cheaper
substitute for marble.
Plate 162 D shows an aggregate of calcareous
piso-liths, spheroidal particles made of concentric shells
of calcium carbonate. The internal structure is magnified in plate 163.
Pisoliths grow in both external and subterranean ponds containing
slightly turbulent water saturated in carbonate. Similar but smaller
particles, called ooliths (or, better, ooids )
are found in agitated waters of the marine realm, more precisely in
marginal areas of carbonate platforms. They derive from stepwise coating
of tiny suspended particles in CO2-rich water currents.
All samples belong to the collection of the Department
of Geological Sciences, University of Bologna.
Photos: G. Piacentini
1970.