|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Introduced Species Summary
Project
West
Nile Virus
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Project
Home | Taxonomy | Identification
| Distribution | Introduction
Facts | Establishment | Ecology | Benefits | Threats | Control |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Name: West Nile Virus
Classification:
Phylum or
Division: Viruses
Class: RNA positive-strand viruses
Family: Flaviviridae
Genus: Flavivirus Japanese Encephalitis Antigenic Complex1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Identification: West Nile Virus consists of
a protein coat or capsid, which surrounds a nucleic acid core of positive-sense,
single stranded RNA of about 10,000 bases. The capsid is contained within
a spherical outer envelope, about 50 nanometers in diameter, composed of
proteins, lipids, trace metals, and carbohydrates; the capsid is half that
diameter. West Nile Virus cycles from avian hosts via arthropod vectors which
are usually mosquitoes. Some instances of a cycle between frogs and mosquitoes
have been discovered; where mosquitoes are absent sometimes ticks can serve
as a vector. West Nile is called an arbovirus, a contraction of arthropod
borne virus. The primary arthropod vector for West Nile
is the mosquito and the primary reservoirs for West Nile are ‘old-world’
bird species. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
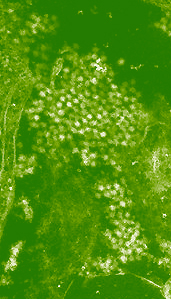
Picture
1. Lime-white circles are West Nile Virus isolated in an electron micrograph
of brain tissue of a crow found in New York. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Original
Distrubution: Flaviviruses have been traced back to a common ancestor
about 15,000 years ago. In 1937 West Nile was first identified2 in the West Nile district of Uganda
from examination of the blood of a woman suffering an high fever. Since then,
West Nile has been isolated in birds, horses, pigs, hamsters, sheep, rhesus
and bonnet monkeys, frogs, mosquitoes, ticks, and humans and discovered to
be the most ubiquitous3 of the Flaviviruses;
it has been found to be endemic to regions from Spain to Asian Russia and
as far south as South Africa and Malaysia.
Current Distrubution:
Probably in all the contiguous United States and, though currently un-reported,
due to avian migratory routes West Nile Virus is being introduced throughout
the Americas. In the United States West Nile has been found in forty mosquito
species and over 70 avian species.4
|
|
|
|
|
|
|
|
|
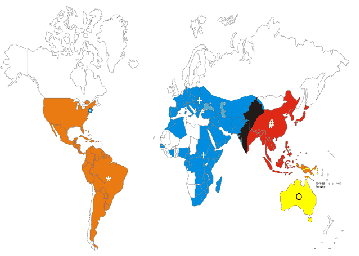
Picture
2. Historical
distrubution of West Nile Virus (blue) and other Flaviviridae: St. Louis
Encephalitis, orange; Japanese Encephalitis, red; West Nile and Japanese Encephalitis,
black; Murray Valley and Kunjin, yellow. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Site
and Date of Introduction: In the summer of 1999 West Nile was introduced
for the first time to North America.
Mode(s) of
Introduction: When crows began to die, suspicions of a possible epizootic were
aroused, but it was not until fall that West Nile Virus was identified, at
which point the (human) epidemic had apparently died out. Ultimately, analysis
of the strain of West Nile in the United States indicated that it was most
closely related to West Nile that had been isolated from an Israeli Goose
in 1998.5 How did West NileVirus
get from Israel to the United States? No one really knows. A mosquito or
tick had to ingest the blood and the West Nile Virus with it from livestock
geese in 1998. Possible vectors include legally or illegally imported flora,
which might harbor the vector or its eggs, and fauna for food or pet trade.
An immune deficient human could have transported the virus to New York City,
so too could mosquitoes trapped in transatlantic airplanes or larvae on transatlantic
boats.
|
|
|
|
|
|
|
|
|
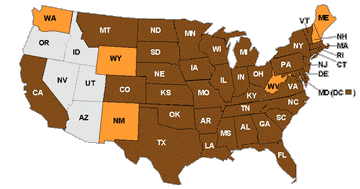
Picture
3. As of 18
November 2002, documented range of West Nile Virus in the United States:
brown states indicate human cases; orange states have only animal, avian,
or mosquito infections |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reason(s)
Why it has Become Established: Infected birds stopover in
the Middle East during their summer migrations. These birds may be infected
from a non-migratory enzootic population of birds probably in northern Africa
or equatorial regions of South Asia. Arthropods, usually mosquitoes, serve
as a vector of transmission of West Nile Virus from the enzootic population
to the migratory population. Since mosquitoes’ reproductive rate and activity
are limited by temperature, other arthropods such as ticks, which are not
so restricted, are frequently vectors in more temperate zones. The enzootic
to migratory transmission reverses itself once the migratory birds stopover
in Israel, or other Eurasian locales, enroute to their northern summer and
breeding grounds. Once the local mosquito population is infected with West
Nile Virus, then it is a matter of time before the local avifauna becomes
infected; if West Nile Virus is novel (as in the Americas) or introduced
in this fashion to a naïve community of birds, some cannot form antibodies
and die; others develop antibodies to the virus or may be genetically predisposed
to resist Flaviviruses. Over time a winnowing process occurs and birds better
able to resist or survive the West Nile Virus infection are able to out-compete
other birds. Concurrently, West Nile Virus, when it replicates its RNA intracellularly,
sometimes makes copying errors. These errors usually do not benefit the virus,
but, since the virus replicates at a rate many times that of the host, one
beneficial error in several thousand secures the reproductive successes of
those viruses with the beneficial error. A seesawing occurs between the virus,
becoming more virulent, and the host species, becoming more resistant. Other
times the virus in replicating itself jumbles proteins: its own and those
of its host. These jumblings infrequently benefit the virus by inserting
host specific protein receptors or even protein receptors common to both
that host and another related species. Thus ‘armed’ West Nile virus may successfully
infect another organism, but only if it is introduced to it out of the mosquito-bird
cycle. This is where humans enter the cycle. |
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
Picture
4. West Nile
Virus Enzootic Cycle: mosquito (vector) to bird (reservoir) and back to mosquito. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By developing a close relationship
with domestic flora and fauna, in this instance livestock, we put ourselves
at risk to infection from epizootic diseases;6
this risk increases exponentially when livestock are transported globally.
The Israeli goose was infected accidentally because of its proximity to an
aviphilic vector carrying West Nile. How then does the virus enter human
populations? Actually it usually does not exit the mosquito-bird cycle excepting
unusual environmental conditions.
As climate change rises global mean temperatures, fluctuations result.7 Throughout the world there are cycles
of drought and rainfall, it is only when egregious droughts occur that endemic
reservoirs for vector species die-off and the vector is forced to human or
livestock hosts. This is compounded as more and more humans enter endemic
systems more frequently. Birds on wing are able to leave the areas where
water is lacking; mosquitoes go dormant some even require a cycling of drought
and moisture for their eggs to hatch. In New York in 1999 there was an unusual
drought. Birds flew away and mosquitoes were forced to the remaining standing
bodies of water most of which were urban. Unattended pools and the plethora
of urban floral attentions, including those at public zoos and gardens, create
ideal habitats for both aviphilic and anthrophilic mosquitoes. When late
summer rain does arrive the mosquito population booms and without one of
their major natural predators, birds, the females, seeking a protein source,
blood, for their eggs, are voracious. At this point transmission is just
a matter of odds: one female mosquito, infected with West Nile and without
an avian source of blood, had to bite and infect one human or even house
pet.
Healthy adult humans usually are asymtomatic to West Nile;8 our immune system prevents buildup
of sufficient numbers of the virus to cause symptoms and more importantly
transmission. This is not the case with children, who are developing immune
responses, and the elderly, who often have a delayed immune response; both
develop a viræmia, which if cycled back into mosquitoes could and at
several points obviously did re-infect humans and birds. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
|
Picture
5. Standing water in an hanging tire swing offers an ideal location for
mosquito larvae. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Picture
6. Mosquito laying an egg-raft in standing water. |
|
|
|
|
|
|
|
|
|
|
|
Ecological Role:
It would be hard to define an ecological role for West Nile Virus.
Perhaps one might say that West Nile Virus acts as a selective mechanism;
it promotes the evolution of species with which it can co-exist.
Benefit(s): Again, it is hard to say. As a biological
or quasi-biological entity, West Nile Virus is as beneficial as a cetacean
or a saurischian; the anthropo-centric labeling of a beneficial species is
a value judgement. Human benefits from West Nile Virus are indirect; the
“outbreak” in New York City resulted in a system of interdisciplinary communication
among scientists, healthcare officials, and administrators; the study of
West Nile Virus might result in a vaccine, which could lead to other Flaviviral
vaccines. West Nile Virus prevention efforts might lead to human re-evaluation
of pesticide and water management.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
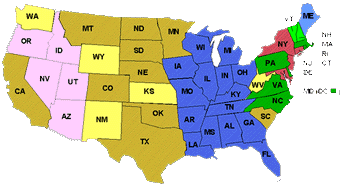
Picture
7. West Nile Virus spread in the United States from 1999 - 2002: 1999,
red;
2000, green; 2001, blue; 2002, yellow; pink states have no reported West
Nile Virus activity in animals, mosquitos, avians, or humans. In each color
category, the darker color is indicative of human cases, i.e. the lighter
colored Maine, Vermont, and New Hampshire have no recorded human West Nile
Virus cases. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Threats: By the end of fall in
1999, West Nile Virus had established itself in the New York City area, since
come the next summer it reappeared; this time, predominantly in Staten Island,
not Queens. There are marsh areas in Staten Island, which, before human interruption
of water cycles, filtered great quantities of water; now there are a lot
of stagnant water bodies in the Staten Island marsh areas; these serve as
breeding grounds for anthropophilic mosquitoes and established a mosquito-human
cycling of West Nile. According to the CDC, West Nile Virus can overwinter
in female Culex mosquitoes.6 A study
published in the Journal of Medical Entomology9
stated that most North American mosquitoes, if infected with West Nile could
transmit the virus. The same study also witnessed the vertical transmission,
from female to eggs, of West Nile in Culex pipiens, the aviphilic
mosquito, which under environmental pressures will switch to humans. In other
words, this is another way West Nile Virus can build up to high concentrations
in bird and mosquito populations. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
West Nile Virus is now found in 43 states
and parts of southern Canada; this is an incredibly rapid spread in just
three years. Birds are extremely efficient means of conveyance for West Nile
as they migrate north and south in the Americas; there are probably as yet
unidentified epizootic occurrences of West Nile virus in South and Central
America. Each newly established bird-mosquito cycling of West Nile allows
the virus to build up to significant concentrations so that should environmental
conditions alter (prolonged dry spells), which the invariably do, the potential
for human and livestock infection is great. The American crow, Corvus
brachyrhynchos, is particularly ill-adapted to launch an immune response
to West Nile and has succumbed in great numbers. Paul Epstein, in the Journal
of Urban Health, proposes7 that C.
brachyrhynchos is ideally situated for infection and re-infection, since
they frequently feed on automobile killed insects and fauna alongside roads
where there are stagnant puddles in drainage ditches: ideal breeding habitats
for mosquitoes. Many other a-synanthropic birds are probably dying, but go
unnoticed since they are in sylvan habitats where they would decompose rapidly.
These American avian species may evolve an immune response to West Nile virus
like the ‘old-world’ birds have done, though if American birds do not they
will become extinct. The USGS conducted a study in 2000 that showed that
West Nile could be transmitted directly from crow to crow.10 If these very social birds are
uniquely predisposed to this form of transmission, which remains to be seen,
then this may as well explain their extreme susceptibility to West Nile.
Some few crows do appear to develop antibodies; they will perhaps in time
give rise to a more resistant populations. |
|
|
|
|

|
|
|
|
|
|
|
Picture
7. The American crow, Corvus brachyrhynchos |
|
|
|
|
|
|
Control Level
Diagnosis: Has West Nile Virus invaded all the Americas? We shall have
to await the coming summer’s tallies of states or countries with human and
faunal infections, bird deaths, and precipitation indices. Currently 176
persons have died in North America from West Nile viral infection; 3,200
or so cases have been officially recorded.11
Actually numbers of infection are probably much higher due to those infected
asymptomatically. West Nile Virus certainly has invaded almost all of the
continental United States, excluding Alaska. There is no feasable way to
stop its spread of West Nile Virus throughout the Americas. Control of zoonotic
spread of West Nile Virus is possible by controlling the vector breeding
spots, much as has been done in the recent past to control malaria.
West Nile Virus is a re-emerging infectious disease12 and has in short order become a
panzootic. The modern world is undergoing a rapid rate of intentional and
unintentional transportation of flora and fauna from endemic to ecdemic ecosystems.
This redistribution is extremely harmful to the functioning of ecosystems
and to local and global human economies.13
14 North and South America
in this vein can be thought of as islands and, applying Wilson and Simberloff’s
theory of Island
Biogeography, as the effective distance from the ‘mainland’, in this
case the ‘old-world’, decreases, the rate of viral invaders increases. West
Nile Virus, AIDS, and many other re-emerging infectious diseases are slated
to be spread about the world by our current intercourse.
Unlike AIDS, West Nile Virus has little direct effect upon humans;
the rate of human infection from the virus is very low. In 1999 during the
first 'outbreak', Despite the virus’ adaptive advantages only about 1% of
mosquitoes carry it and only about 1% of humans bitten develop any symptoms,
which are usually mild fevers.10
Only an estimated 20% of the 1% bitten develop serious symptoms such as encephalitis
or meningitis.15 Despite the media
brouhaha, humans are not significantly affected by the introduction of West
Nile to North America, but other vertebrates and arthropods seem to be affected
in much great numbers. Around 80% or more of the reported avian deaths since
West Nile’s introduction have been American crows.9 No studies have yet been conducted
upon a-synanthropic native avifauna. Perhaps the effect of West Nile Virus
is sufficient to extinguish endemic birds already severely stressed by habitat
losses. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
Picture
8. Female mosquito feeding on human (only female mosquitoes require blood). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Control Methods: Unfortunately
attempts to control West Nile Virus by insecticide are significantly hazadous
to the health of all living organisms and cause environmental and human health
costs far in excess of any damage that West Nile Virus may incur if left
un-checked. Since West Nile virus relies upon a vector for transmission,
then control of the vector will limit the zoonotic transmission. This means
no standing water and cessation of herbicide, pesticide, and other chemical
applications, which destroy the mosquitoes' endemic predators: birds, fish,
and insects. When the West Nile Virus appeared in New York City in the summer
of 1999, Mayor Rudolph Giuliani and City Health Officials reacted swiftly
in authorizing nighttime blanket spraying of the city.16 According to the New York City
Green Party 2001 mayoral candidate, Mitchel Cohen, the city sprayed “a toxic
mix of Fyfanon ULV (96.5 percent pure Malathion) from helicopters and the
synthetic Pyrethroids Scurge
and Anvil
(Resmethrin and Sumithrin, which also contained large amounts of ‘helper’
chemicals such as Piperonyl Butoxide, an FDA-listed human cancer-causing
agent).”17 For more on
the use of pesticides in New York City and the control of West Nile Virus
see the webpage:
Is Silent Spring Relevant Today? |
|
|
|

|
|
|
|
|
Picture
9. Airplane spraying pesticide over unknown city. Recent pesticide spraying
in New York was done from trucks on the ground |
|
|
|
|
|
|
|
|
|
|
References:
1. This complex includes Alfuy, Capipacore, Japanese
Encephalitis, Kunjin, Murray Valley Encephalitis, St. Louis Encephalitis,
Rocio, Usutu, Stratford, and Kyasanur viruses. CDC.
2. Petersen, Lyle R. and Roehrig, John T.
West Nile Virus: A reemerging global pathogen. Review of Biomedicine
2001. 12:208-216.
3. Hubálek, Zdnek and Halouzka, Jirí.
West Nile Fever—a Reemerging Mosquito-Borne Viral Disease in Europe.
Emerging Infectious Diseases 1999. 5(5).
4. Enserink, Martin. West Nile’s Surprisingly
Swift Continental Sweep. Science 2002. 297:1988-1989. Also see Rutgers
site.
5. Lanciotti, R.S. etal. Origin of the
West Nile Virus Responsible for an Outbreak of Encephalitis in the Northeastern
United States. Science1999. 286:2333-2337.
6. Centers for Disease Control. Update: Surveillance
for West Nile virus in Overwintering Mosquitoes. Morbidity and Mortality
Weekly Report 2000. 49:178-179.
7. Epstein, Paul R. West Nile Virus and
the Climate. Journal of Urban Health: Bulletin of the New York Academy
of Medicine 2001. 78(2):367-371.
8. Despommier, Dickson. West Nile Story:
A New Virus in the New World. 2001. New York: Apple Tree Productions.
pp. 10-20.
9.Turell, Michael J. etal. Vector Competence
of North American Mosquitoes (Diptera: Culicidae) for West Nile Virus.
Journal of Medical Entomology 2001. 38 (2): 130-134.
10. USGS
Press Release. 26 October 2000.
11. The National Biological Information
Infrastructure. 22 October, 2002.
12. Daszak, Peter, etal. Emerging Infectious
Diseases of Wildlife–Threats to Biodiversity and Human Health. Science
2000. 287:443-449.
13.Vitousek, Peter, etal. Biological
Invasions as Global Environmental Change. American Scientist 1996. 84:468-478.
14. Pimentel, David, etal. Economic and
Environmental Threats of Alien Plant, Animal, and Microbe Invasions.
Agriculture, Ecosystems, and Environment 2001. 84:1-20.
15. CDC.
22 October, 2002.
16. Questions and Answers
about West Nile. NYC Department of Health. October 2000.
17.Mitchel. It
should be noted that Fyfanon ULV is extremely acidic. According to CHEMNOVA,
its manufacturer, Fyfanon ULV has a pH of 3.7 - 3.8 when combined with equal
amounts of distilled water and dispersed at 20&Mac251;C.; this really
is strong enough to strip paint off of automobiles!
Photo and
Picture Credits:
1. CDC
2. CDC
3. CDC
4. Woindstar
5. Dutchess County
6. PBS&J
7. Henrico County, Virginia,
Health Department
8. Blue Ridge
Family Health Center
9. University
of Buffalo Libraries
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Author: Aleksei Chmura
Last Edited: 19/11/02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|










