40. Red cabbage juice, a natural product, can be used as an acid-base indicator:
41. Neutralization Reactions: with 0.10 M HCl solutions.
42. Titration reactions:
43. Buffer Solutions: Adding relatively small amounts of strong acid or strong base solutions to water markedly changes the pH. In fact simple dilution is sufficient to change the concentration and, therefore, the pH, substantially. But buffered solutions are just that... buffered... and can absorb significant amounts of acid or base without much change in pH. Here, acid and base are added to buffered solutions containing a visible indicator.
44. Metallic oxides form alkaline solutions due to hydroxide ion formation:
Calcium oxide: CaO(s) + H2O(liq) 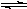 Ca(OH)2(aq)
Ca(OH)2(aq) ![]() Ca2+(aq) + 2 OH-(aq)
Ca2+(aq) + 2 OH-(aq)
45. Nonmetal oxides form acidic solutions due to hydronium ion formation:
46. Metallic hydrides form alkaline solutions due to hydroxide ion formation:
Sodium hydride:
2 NaH(s) + H2O(liq) ![]() 2 Na+(aq) + 2 OH-(aq) + H2(g)
2 Na+(aq) + 2 OH-(aq) + H2(g)
47. Railroad and "regular" chalk
48. Hydrolysis of Salts: Each of the following salts dissolve in water and the pH of the resulting aqueous solutions is a function of the hydrolytic (hydrolysis) reaction with the anions and/or cations that are formed:
49.Conductivity is demonstrated in water, glucose and salt solutions; and in aqueous ammonia and acetic acid solutions. Behavior was first understood by Arrhenius in terms of the presence of ions as carriers of charge.
50. Amphoteric (amphiprotic) substances: Acting as an acid or a base, zinc hydroxide undergoes the following reactions:
51. Sparingly Soluble Salts: Aqueous potassium chloride, bromide and iodide solutions are (separately) added to aqueous silver nitrate solutions, precipitating the sparingly soluble silver halides. The resulting precipitates are then treated with aqueous ammonia and, in the case of the iodide, with cyanide ion.
52. Neutralization Reactions: A light bulb is used as a visible indicator for the neutralization of a sulfuric acid solution with barium hydroxide. The product of the reaction is the sparingly soluble salt, barium sulfate.
53. Complex ion formation: Co2+ and Ni2+ ions form complex ions known as coordination complexes with ammonia (NH3), ethylenediamine (H2NCH2CH2NH2), and cyanide ion (CN-), which are referred to as ligands. Note that the diamine has two coordination sites (bidentate ligand) while the ammonia and the cyanide have only one site (monodentate ligands).
54. Common Ion Effect: Adding concentrated HCl solution to a saturated solution of salt in water has the effect of diminishing the solubility of the salt.