CaO(s) + H2O(liq) 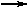 | Ca(OH)2(s) + heat | DH<0 |
| quicklime | slaked lime |
Demonstrations for Chapter 10: Thermochemistry
54. Exothermic reactions give off heat to the surroundings: DH<0; sign is (-). Jack's demonstration uses heat from the hydration of quicklime, producing "slaked" lime, to fry an egg:
CaO(s) + H2O(liq) 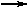 | Ca(OH)2(s) + heat | DH<0 |
| quicklime | slaked lime |
55. Endothermic reactions absorb heat from the surroundings: DH>0; sign is (+ Jack's demonstration makes use of the reaction between ammonium thiocyanate and barium hydroxide to freeze water):
heat + NH4SCN (s) + Ba(OH)2 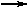 NH3 + 2 H2O + Ba(SCN)2 NH3 + 2 H2O + Ba(SCN)2 |
DH>0 |
56. The "alcohol cannon" simulates the explosive events in a compression chamber of an internal combustion energy. The two nails act as electrodes.
57. Pyrotechnic display:
58. Sugar and sulfuric acid. The dehydration reaction is violently exothermic, leading first to carmelization, and then carbonization.
59. Useful work performed by expanding gases: poping a rubber stopper; raising a book.