H2(g) + 1/2 O2(g) 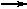 H2O(liq)
H2O(liq) | DH = -68.3 kcal |
CaO(s) + H2O(liq) 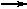 Ca(OH)2(s) Ca(OH)2(s) | DH = -15.3 kcal |
Ca(s) + 1/2 O2(g) 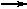 CaO(s) CaO(s) | DH = -151.8 kcal |
Fe2O3(s) + 3 CO(g)
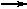 3 CO2(g) + 2 Fe(s) Ans: -24.77 kJ
3 CO2(g) + 2 Fe(s) Ans: -24.77 kJ2 NO2(g)
 2 NOg) + O2(g) Ans: 114.14 kJ
2 NOg) + O2(g) Ans: 114.14 kJN(g) + NO(g)
 N2(g) + O(g) Ans: -313.78 kJ
N2(g) + O(g) Ans: -313.78 kJ C2H6(g)
C2H6(g)C3H6(g) + H2(g)
 C2H6(g)
C2H6(g)
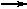 CnH2n+2(g)
CnH2n+2(g)