ChemBytes - Chapter 7
February 8, 1998
CHEMByte 11: Equilibrium, Limestone, Mortar... and Herod's Temples.
Understanding and applying Le Chatelier's principle is central to maximizing the yields of desired chemical products in the laboratory and in industry. One extraordinary example is the highly temperature-dependent decomposition reaction of limestone, CaCO3 (s), in the manufacture of calcium oxide (CaO), or quicklime:
CaCO3 (s)  CaO(s) + CO2 (g)
CaO(s) + CO2 (g)
No significant yield of CaO(s) can be obtained at room temperature. However, since the reaction is endothermic, almost complete conversion to products can be achieved by raising the temperature. Quicklime, or just simply lime (CaO) for the construction industry is manufactured from calcium carbonate in this manner. Lime was the fourth highest-volume chemical produced in the United States (1992) with most of it being used in various materials of construction.
Among the most interesting and unique construction projects of all time were the temples, municipal buildings and walled cities built by King Herod the Great, throughout the Near East, notably in what is today the nation of Israel. There was (and still is) plenty of limestone in Israel. However, there wasn't much in the way of wood to fuel the kilns to drive the reaction for manufacturing lime, the traditional and essential agent in mortar, which is an adhesive or bonding agent used since early recorded history. Then as now, mortar was a mixture of various local ingredients including straw, gravel, sand.... and of course, lime... and water. This mixture was first worked into a soft, pliable putty-like material, which set to a hard, infusible binder that was just right for building walls for cities and temples. But by Herod's time there was little in the way of wood left in the Near East, certainly not on the scale of Herod's visions... and therefore there was no way to drive the chemical equilibrium to the desired product. Herod's solution to the problem of no lime was to build his temples without mortar by simply cutting, moving and stacking blocks of monumental size. The Western Wall in the old city of Jerusalem provides us with a good example. Here one can see the massive ashlar blocks which typically weighed 40-50 tons each, neatly cut and carefully laid down one on top of the other. Herod was forced to build without the benefits of mortar for not being able to drive the calcium carbonate equilibrium to the right and produce the needed lime.
CHEMByte 12: Hemoglobin, Oxyhemoglobin, and Carboxyhemoglobin.
The hemoglobin-oxyhemoglobin system carries oxygen in the blood and is one of the most delicately tuned equilibria in nature. It involves a reaction in which the ratio of the oxygenated and deoxygenated products depends directly on the oxygen partial pressure and the value of the equilibrium constant. When both oxygen and carbon monoxide are present the carbon monoxide competes more effectively for the hemoglobin molecule, explaining the lethal character of CO. For example, more than 250 deaths are reported each year from faulty space heaters that produce odorless carbon monoxide gas that quietly kills at night while people sleep. Tennis star Vitas Gerulaitis died in just such an accident.
Hemoglobin is the main chemical component of red blood cells, carrying oxygen by means of the arterial system from the lungs to the body tissues, that is, transporting oxygen from a location of high oxygen concentration to a place of oxygen deficiency. This is accomplished through the formation of an oxygen-hemoglobin complex, oxyhemoglobin:
hemoglobin + O2  oxyhemoglobin
oxyhemoglobin
Hb(aq) + O2(g)  HbO2(aq)
HbO2(aq)

Le Chatelier's principle predicts the equilibrium will shift to the right side of the equation if the oxygen partial pressure increases. This is the situation in the lungs. A shift to the left, releasing the O2, will occur if there is a decrease in the availability of oxygen, as in body tissue. At oxygen pressures commonly found in the lung alveoli, hemoglobin exists as 90-95 percent oxyhemoglobin. At lower pressures of O2, the percent of oxyhemoglobin decreases.
The normal solubility of oxygen in the aqueous medium that is blood is insufficient for physiological function. In the absence of the oxygen-carrying properties of hemoglobin, a liter of blood at body temperature dissolves no more than 3 mL of oxygen; in the presence of hemoglobin, oxyhemoglobin provides the means for oxygen transport, and about 70 times that amount can be dissolved.
Carbon monoxide combines with hemoglobin, forming carboxyhemoglobin, poisoning the oxygen transport capacity of the blood. It was present in the air that Gerulaitis breathed from a defective ventilating system that failed to remove the combustion products of the propane-fueled space heater in his cottage. Obvious effects of high CO levels on health begin at 50 ppm (parts per million) with the inability to distinguish time intervals, drowsiness and nausea; at 100 ppm, distinct cardiac and pulmonary functional changes are observable; and at 250-750 ppm, unconsciousness and eventually death.
Carbon monoxide adds to hemoglobin in the same way oxygen does but exhibits a greater affinity, which means that K(CO) is greater than K(O2):
Hb(aq) + O2(g)  HbO2(aq) K(O2)
HbO2(aq) K(O2)
Hb(aq) + CO(g)  HbCO(aq) K(CO)
When hemoglobin is exposed to a mixture of both oxygen and carbon monoxide, there is a competition for the hemoglobin molecule according to the following equation which results from reversing the first reaction and adding it to the second:
HbO2(aq) + CO(g)
HbCO(aq) K(CO)
When hemoglobin is exposed to a mixture of both oxygen and carbon monoxide, there is a competition for the hemoglobin molecule according to the following equation which results from reversing the first reaction and adding it to the second:
HbO2(aq) + CO(g)  HbCO(aq) + O2(g)
HbCO(aq) + O2(g)
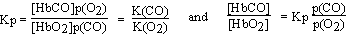
The relative proportions of hemoglobin combined with carbon monoxide and oxygen will be proportional to the partial pressures of the gases present. We know that Kp is greater than one since K(CO) is greater than K(O2). In fact, at 38°C, the value for Kp is 210 and so the position of the equilibrium sharply favors the carbon monoxide complex over the oxygen complex. The figure helps one understand the lethal nature of the gas. These data also demonstrate that the poisoning of a person by inspiration of air containing carbon monoxide can be reversed and inhibited by the use of pure oxygen at a high partial pressure. In other words, a high concentration of oxygen in the blood and lungs will, according to Le Chatelier's principle, displace carbon monoxide from carboxyhemoglobin. The carbon monoxide is simply expired.
The respiration mechanism and the reversibility of the system can be demonstrated with a solution of hemoglobin. Shaking with oxygen turns it the bright red color commonly associated with arterial blood, but when the oxygen is pumped off, the hemoglobin solution assumes instead the bluish-red color characteristic of venous blood.
CHEMByte 13: The Manufacture of Ammonia
Le Chatelier's principle and an understanding of gas phase equilibria have been useful in the industrial preparation of many substances, most importantly, the successful production of ammonia on an industrial scale. Ammonia is an essential chemical intermediate in manufacturing nitrogen-containing compounds including fertilizers, explosives, and other products. Before World War I the traditional precursor for these products had been Chilean nitrates, particularly sodium nitrate (NaNO3). By the beginning of the 20th century, Chilean nitrates supplied two-thirds of the world's industrial and agricultural needs for nitrogen compounds. At the same time, there were obvious concerns about depletion of these resources and about being dependent on one source of supply.
Fritz Haber worked out the chemistry and Karl Bosch did the engineering needed to produce ammonia inexpensively from the elements. The exothermic reaction converts 4 mol of gaseous reactants to 2 mol of gaseous products
3 H2(g) + N2 (g)  2 NH3 (g)
Applying Le Chatelier's principle leads to two conclusions. First, raising the pressure increases the yield of NH3. Second, raising the temperature decreases the yield of NH3. However, the rate of the reaction is very slow, and since raising the temperature generally increases the rate of most chemical reactions, the temperature effect seems to be in conflict with any hope of obtaining a successful process for ammonia synthesis.
In the early years of the 20th century, Haber, a physical chemist and professor at the German University of Karlsruhe, studied this reaction carefully. During nearly a decade of work, Haber and his students found that direct combination of the elements at a high pressure of 500 atm and a moderate temperature of 450°C in the presence of a catalyst, speeded the reaction and improved the yield. They had succeeded in favorably shifting the equilibrium in the right direction as well as speeding it up -- just what was needed for an economical manufacturing process. It took another five years to produce the ammonia inexpensively. By 1913 the first major Haber process plant went "on-stream," beginning continuous operations.
The Haber process was an immediate success. After World War I, the ammonia manufacturing industry grew rapidly. Today, there are some 335 active synthetic ammonia plants worldwide., Although this process has been the subject of considerable modification over the years, the central issue is still the trade-offs between pressure and temperature that favor ammonia formation from the elements, along with the effective use of an efficient catalyst.
The raw materials for the production of ammonia are nitrogen, separated from liquid air, and hydrogen, which is usually produced from a hydrocarbon, a compound of carbon and hydrogen. A high pressure reaction between hydrogen and nitrogen over an iron catalyst produces the ammonia product which is removed as liquid by refrigeration:
3 H2(g) + N2 (g)
2 NH3 (g)
Applying Le Chatelier's principle leads to two conclusions. First, raising the pressure increases the yield of NH3. Second, raising the temperature decreases the yield of NH3. However, the rate of the reaction is very slow, and since raising the temperature generally increases the rate of most chemical reactions, the temperature effect seems to be in conflict with any hope of obtaining a successful process for ammonia synthesis.
In the early years of the 20th century, Haber, a physical chemist and professor at the German University of Karlsruhe, studied this reaction carefully. During nearly a decade of work, Haber and his students found that direct combination of the elements at a high pressure of 500 atm and a moderate temperature of 450°C in the presence of a catalyst, speeded the reaction and improved the yield. They had succeeded in favorably shifting the equilibrium in the right direction as well as speeding it up -- just what was needed for an economical manufacturing process. It took another five years to produce the ammonia inexpensively. By 1913 the first major Haber process plant went "on-stream," beginning continuous operations.
The Haber process was an immediate success. After World War I, the ammonia manufacturing industry grew rapidly. Today, there are some 335 active synthetic ammonia plants worldwide., Although this process has been the subject of considerable modification over the years, the central issue is still the trade-offs between pressure and temperature that favor ammonia formation from the elements, along with the effective use of an efficient catalyst.
The raw materials for the production of ammonia are nitrogen, separated from liquid air, and hydrogen, which is usually produced from a hydrocarbon, a compound of carbon and hydrogen. A high pressure reaction between hydrogen and nitrogen over an iron catalyst produces the ammonia product which is removed as liquid by refrigeration:
3 H2(g) + N2 (g)  2 NH3 (g)
Without the catalyst, the Haber ammonia is no process at all requiring a time frame on a near-geological scale to achieve calculated equilibrium concentrations of product.
2 NH3 (g)
Without the catalyst, the Haber ammonia is no process at all requiring a time frame on a near-geological scale to achieve calculated equilibrium concentrations of product.
CHEMByte 14: Haber, Bosch and Bergius - The Ammonia Chemists.
Fritz Haber (1868-1934) and Karl Bosch (1874-1940) are primary examples of the pre-eminence of German science and the impact of the happy marriage of industrial and academic research on that deserved reputation. The German dye chemists, the emerging drug and pharmaceutical chemists.... and the ammonia chemists helped make it possible. It was during the half-century that ended with the beginning of WWII.
Haber's chemical father was August Wilhelm von Hofmann, under whom he did his doctoral work at the University of Berlin. That made Haber a chemical grandson of the great Justus von Liebig who had established nothing less than the way modern chemistry is taught professionally to this day at his laboratory at Giessen. Under Liebig, Hofmann had worked on chemistry that eventually opened the world to synthetic dyes, and eventually to drugs and pharmaceuticals... and virtually all of the field known as synthetic organic chemistry. Haber received his Ph.D. in 1891 from the aging von Hoffman (who died the next year). By 1898 we find Haber as professor at the University of Dahlem (Berlin), which eventually became the Kaiser Wilhelm Institute, where he began studies in the rapidly emerging field of experimental physical chemistry being built upon the work of Friedrich Ostwald, Professor at Leipzig. Haber's electrochemical studies produced a glass electrode much like the standard glass electrode used today to measure pH by detecting the electric potential across the surface of a thin glass bulb containing a standard acid solution. Being a postdoctoral student with Bunsen at Heidelberg, it is not surprising to find he was also interested in flames and combustion processes... and reactions in the gas phase, which led to his affiliation with the burgeoning German chemical giant Badische Anilin-Soda-Fabrik AG (BASF) and to the synthesis of ammonia.
Karl Bosch received his Ph.D in 1898 at Leipzig under Johannes Wislicenus, the Harvard educated German chemist who first discovered the two isomeric forms of lactic acid, anticipating (and making possible) important work by van't Hoff and Pasteur. Bosch, who had been trained as an engineer before his Ph.D. training, found himself with an especially interesting problem in 1909 as the BASF chief of chemistry and engineering - namely the development of Haber's ammonia process. His job? Nothing less than bringing the laboratory process "on stream," scaling it up to industrial operation. Whereas Haber had used a carbon steel cylinder to carry out the gas phase reaction, Bosch switched to an alloy steel, thus preventing the embrittlement and eventual failure that took place over time due to the high pressures and temperatures required. Under Bosch's management and direction, a gigantic Haber ammonia process plant was built at Oppau just as WWI began. Since Haber ammonia is just as important for explosives manufacturing as for fertilizer applications, it was not surprising that this plant served the German war effort before it ever began its intended peaceful use -- fertilizer ammonia -- for which Bosch shared the 1931 Nobel Prize in chemistry with Friedrich Bergius (1884-1949).
Bergius was a 1907 Ph.D. student of Haber (Leipzig) and an expert on high pressure gas phase and catalytic processes. Besides his work on Haber ammonia, he is given the lion's share of credit for his first successful commercial processes for converting coal and crude oil to useful forms of gasolines for automotive and aviation fuels. To a considerable extent, these chemical studies helped supply Germany in WWII.
CHEMBytes: Additional Problems on Equilibrium
- The equilibrium constants for the following reactions have been measured at 823K:
CoO(s) + H2(g)
 Co(s) + H2O(g)
K1 = 67
Co(s) + H2O(g)
K1 = 67
CoO(s) + CO(g)  Co(s) + CO2(g) K2 = 490
From these data, calculate the equilibrium constant of the following reaction at 823K:
CO2(g) + H2(g)
Co(s) + CO2(g) K2 = 490
From these data, calculate the equilibrium constant of the following reaction at 823K:
CO2(g) + H2(g)  CO(g) + H2O(g) K3 = ?
CO(g) + H2O(g) K3 = ?
- Solid ammonium carbamate, NH4COONH2, dissociates completely into ammonia and carbon dioxide when it evaporates, as shown by the following equation:
NH4COONH2(s)
 2 NH3(g) + CO2(g)
At 25°C, the total pressure of the gases in equilibrium with the solid is 0.116 atm. What is the equilibrium constant of the reaction? If 0.10 atm of CO2 is introduced after equilibrium is reached, will the final pressure of CO2 be greater, less than, or equal to 0.1 atm? Will the pressure of NH3 increase, decrease, or stay the same?
2 NH3(g) + CO2(g)
At 25°C, the total pressure of the gases in equilibrium with the solid is 0.116 atm. What is the equilibrium constant of the reaction? If 0.10 atm of CO2 is introduced after equilibrium is reached, will the final pressure of CO2 be greater, less than, or equal to 0.1 atm? Will the pressure of NH3 increase, decrease, or stay the same?
- The gaseous compound NOBr decomposes according to the reaction
NOBr(g)
 NO(g) + 1/2 Br2(g)
At 350K, the equilibrium constant Kp is equal to 0.15. If 0.50 atm of NoBr, 0.40 atm of NO, and 0.20 atm of Br2 are mixed at this temperature, will any net reaction occur? If so, will Br2 be consumed or formed?
NO(g) + 1/2 Br2(g)
At 350K, the equilibrium constant Kp is equal to 0.15. If 0.50 atm of NoBr, 0.40 atm of NO, and 0.20 atm of Br2 are mixed at this temperature, will any net reaction occur? If so, will Br2 be consumed or formed?
- 4. Would you expect the equilibrium constant for the following reaction to increase, decrease, or stay the same as the temperature increases? Why?
I2(g)
 2I(g)
2I(g)