Exam I Chemistry G8248 Part II
Monday, December 9, 1996
Instructions: This is a one hour, closed book examination.
All material to be graded must be placed in a blue book with your name on the front cover.
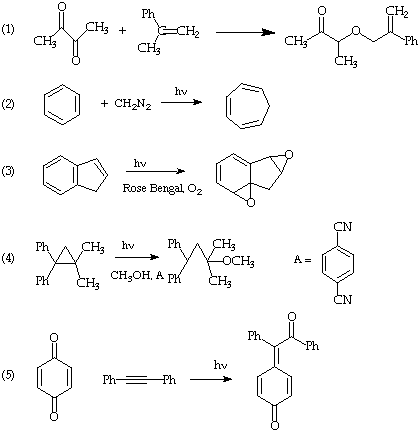
I. (30 points). Suggest mechanisms which rationalize THREE of the following photochemical transformations. In each case specify explicity the primary photochemical step. Use only primary photochemical and secondary processes that have precedent in the class lectures or in Modern Molecular Photochemistry. If you cannot use a precedent, so indicate.

II. (10 points). Assume a reaction coordinate involving a coplanar electron abstraction initiated by the n-orbital of a n,
p* state of a ketone attacking an amine. Draw a state correlation diagram for the electron transfer process, indicating how S1(n,p*) and T1(n,p*) are correlated to the primary products of the electron transfer. Is this reaction photochemically allowed in S1(n,p*)? Is this reaction photochemically allowed in T1(n,p*)?
III. (10 points). Explain qualitatively the observation that photolysis of dibenzyl ketone in an NMR spectrometer results in a 13-C NMR spectrum that consists of emission at a resonance position corresponding to the carbonyl carbon of DBK and absorption corresponding to carbon monoxide.
IV. (10 Points). The photolysis of both A and B in the presence of toluene results in good yields of hydrogen abstraction products. However, the rate of reaction of A with toluene and perdeuterated toluene shows a significant isotope effect (the deuterated toluene reacts much slower), while the rate of reaction of B with toluene and deuterated toluene proceed without an isotope effect. Why is there an isotope effect for A but not for B?
V. (10 Points). Irradiation of A in methanol in the presence of acetone results in the addition of methanol selectively to only one of the double bonds. To which double bond does the methanol add? Provide an explanation of the selective addition.
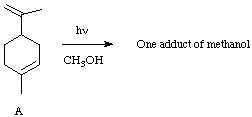
VI. (10 Points) Photolysis of acetaldehyde leads to the same set of products as the photolysis of acetoin (both in a photochemically inert solvent), a characteristic of reactions involving a common intermediate(s). Suggest three products that might result from common reaction intermediates produced from each photolysis and show how these product result from a common intermediate(s).

VII. (10 Points) Photochemical excitation of cyclobutanone in methanol results in a high yield of the acetal shown. Write a mechanism for the formation of the acetal which is consistent with the standard primary photochemical processes of n,
p* states.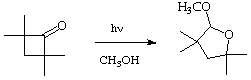
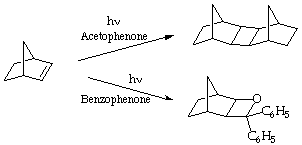
VIII. (10 Points) The photolysis of norbornene in the presence of acetophenone results in the formation of norbornene dimer(s), whereas the photolysis of norbornene in the presence of benzophenone results in the formation of an oxetane. In both cases the ketone, not the norbornene are excited photochemically. Explain why the photolysis follows different pathways.