EXAM I Chemistry G8298y
Friday, March 12, 1993
Instructions: This is a one hour, closed book examination.
All material to be graded must be placed in a blue book with your name on the front cover.
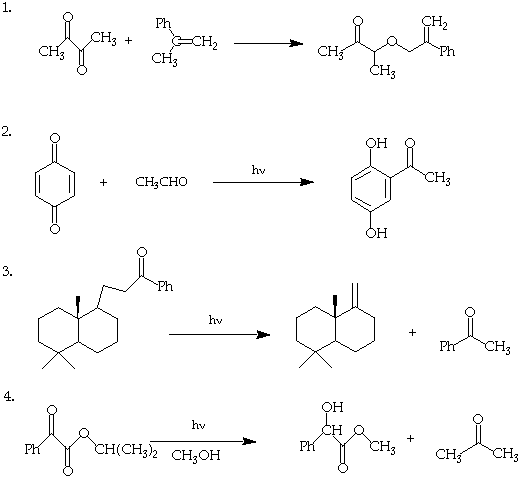
I. (40 Points). Suggest mechanisms which rationalize the following photochemical transformations. In each case specify explicity the primary photochemical step. Use only primary photochemical and secondary processes that have precedent in the class lectures or in Modern Molecular Photochemistry. If you cannot use a precedent, so indicate.

II. (30 points). Suggest five products that you would expect to be produced from photolysis of the following molecules.
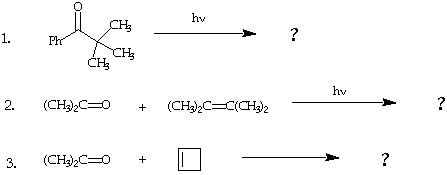
III. (10 points). Assume a reaction coordinate involving a coplanar electron abstraction initiated by the n-orbital of a n,
p* state of a ketone attacking an amine. Draw a state correlation diagram for the electron transfer process, indicating how S1(n,p*) and T1(n,p*) are correlated to the primary products of the electron transfer. Is this reaction photochemically allowed in S1(n,p*)? Is this reaction photochemically allowed in T1(n,p*)?
IV. (10 points). As discussed in class, chemically induced dynamic nuclear polarization (CIDNP) of the products of radical pairs is commonly observed when ketones are photolyzed in an NMR spectrometer. Although 1,4-biradical intermediates are presumed to be involved in many photoreactions of ketones (e.g., Type II hydrogen abstraction and n-orbital initiated addition to C=C bonds), chemically induced dynamic nuclear polarization (CIDNP) of the products of 1,4-biradicals is generally not observed when these intermediates are produced by photolysis in a NMR spectrometer. Suggest two different reasons why CIDNP is not expected to be observed from the products of 1,4-biradicals.
V. (10 points). Explain qualitatively the observation that photolysis of dibenzyl ketone in an NMR spectrometer results in a 13-C NMR spectrum that consists of emission at a resonance position corresponding to the carbonyl carbon of DBK and absorption corresponding to carbon monoxide.