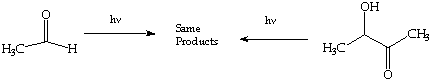
EXAM I Chemistry G8298y
Tuesday, May 10, 1994
Instructions: This is a one hour, closed book examination.
All material to be graded must be placed in a blue book with your name on the front cover.
1. (10 Points) Photolysis of acetaldehyde leads to the same set of products as the photolysis of acetoin (both in a photochemically inert solvent), a characteristic of reactions involving a common intermediate(s). Suggest three products that might result from common reaction intermediates produced from each photolysis and show how these product result from a common intermediate(s).
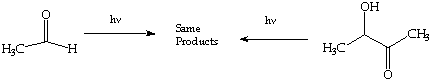
2. (20 Points)
(a) Construct the state energy diagram for the photochemically induced electron abstraction reaction between formaldehyde and ammonia. Assume that the reaction proceeds via the interaction of the n-orbital of the n,
(b) Based on your energy diagram, is the reaction allowed from the T
1 (n,p*) state? From the S1 (n,p*) state?
3. (10 Points) Photochemical excitation of cyclobutanone in methanol results in a high yield of the acetal shown. Write a mechanism for the formation of the acetal which is consistent with the standard primary photochemical processes of n,
p* states.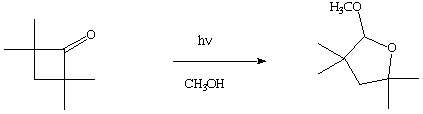
4. (10 Points) The photolysis of dibenzyl ketone, especially in micellar solutions, results in the 13-C enrichment of the recovered, partially reacted dibenzyl ketone. The efficiency of enrichment is magnetic field dependent. Do you expect the efficiency of 13-C enrichment to increase or decrease in a strong magnetic field? Give an explanation for your answer.
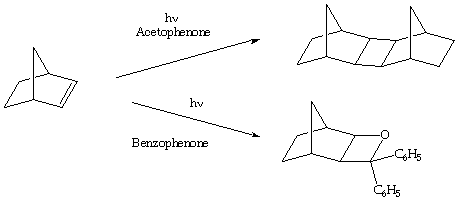
5. (10 Points) The photolysis of norbornene in the presence of acetophenone results in the formation of norbornene dimer(s), whereas the photolysis of norbornene in the presence of benzophenone results in the formation of an oxetane. In both cases the ketone, not the norbornene are excited photochemically. Explain why the photolysis follows different pathways.
6. (10 Points) The photolysis of the
a,a'-dipropylcyclohexanone shown below results in the exclusive formation of the epimer shown. Suggest a reason why this product, rather than the alternative epimer is formed.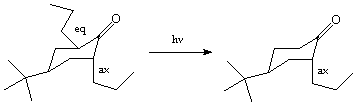
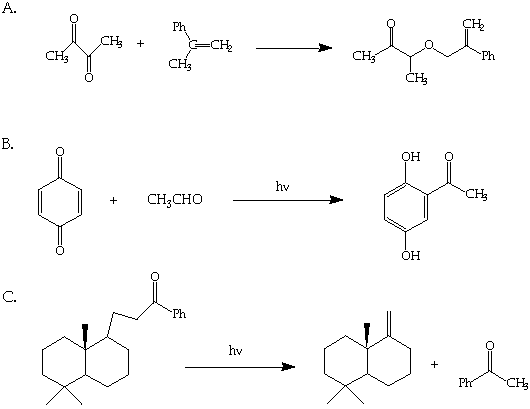
7. (30 Points; 10 Points each part) Suggest mechanisms for the following photochemical transformations.