EXAM II Chemistry G8298y
Friday, April 30, 1993
Instructions: This is a one hour, closed book examination.
All material to be graded must be placed in a blue book with your name on the front cover.
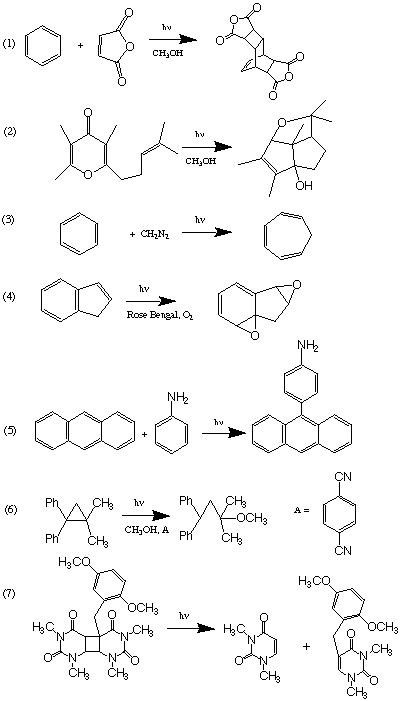
I. (50 Points). Suggest mechanisms which rationalize five of the following photochemical transformations. In each case specify explicitly the key reactive intermediates you think are needed to rationalize the results. Use only primary photochemical and secondary processes that have precedent in the class lectures or in Modern Molecular Photochemistry. If you cannot use a precedent, so indicate.

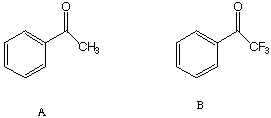
II. (10 Points). The photolysis of both A and B in the presence of toluene results in good yields of hydrogen abstraction products. However, the rate of reaction of A with toluene and perdeuterated toluene shows a significant isotope effect (the deuterated toluene reacts much slower), while the rate of reaction of B with toluene and deuterated toluene proceed without an isotope effect. Why is there an isotope effect for A but not for B?
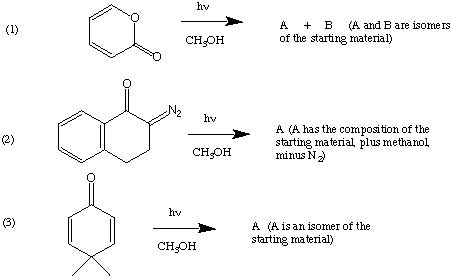
III. (30 Points). Suggest products you expect to result from the following photochemical transformations.
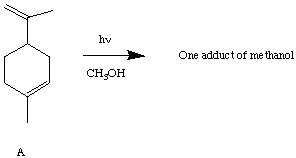
IV. (10 Points) Irradiation of A in methanol in the presence of acetone results in the addition of methanol selectively to only one of the double bonds. To which double bond does the methanol add? Provide an explanation of the selective addition.