Exam I Chemistry G8248
Friday, November 18, 1994
Instructions: 50 minutes. Closed book exam.
All material to be graded must appear in your blue book clearly numbered corresponding to the number of the question you are answering. Show your reasoning for full or partial credit.
1. (20 points). A molecule displays the following emission parameters at 77
oK: FF = 0.50 tS = 10-7 sec FST = 0.50 tT = 1.0 sec FP = 0.05Show a state diagram for this molecule and indicate on the diagram the values of k
F, kST, kIC, kP and kTS.Suggest a plausible structure that is consistent with these data and suggest a structure that is inconsistent with these data.
2. (10 points). Benzophenone (S
1 and T1 = n,p*) undergoes intersystem crossing from S1 with a rate constant kST ~ 1011 sec-1. Acetone (which also possesses S1 and T1 n,p* states) undergoes intersystem crossing from S1 with a rate constant kST ~ 108 sec -1. Both molecules undergo intersystem crossing from T1 with comparable rate constants (kTS ~ 102 sec -1). Suggest a rationale for the difference of 3 orders os magnitude in kST for the two ketones. Suggest a rationale for the comparable magnitude of kTS of the two ketones.
3. (20 points). Give an estimate of the magnitude of the following quantities. Be sure to note units explicitly and clearly.
(a) The largest value plausible for:
(i) the radiative rate constant for fluorescence of an organic molecule consisting of only atoms in the second row of the periodic table;
(ii) the radiative rate constant for phosphorescence of an organic molecule;
(iii) the bimolecular rate constant for triplet-triplet energy transfer.
(ii) the radiative rate constant for phosphorescence of an organic molecule.
4. (20 Points). Draw the state correlation diagram for the Type II hydrogen abstraction of 2-hexanone (1) and use the diagram to rationalize the following observations:
The efficiency of hydrogen abstraction from the singlet state of 1 is low (ca 10%), while the efficiency of hydrogen abstraction from the triplet state of 1 is high (ca 100%).
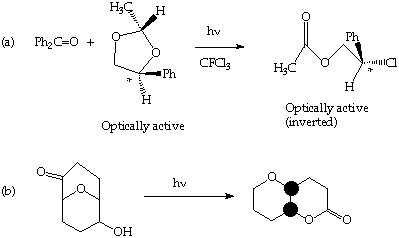
5. (20 Points). Suggest a plausible mechanism for the following photochemical transformations.
