

Jeffrey T. Koberstein
Percy and Vida Hudson Professor of Chemical Engineering
Columbia University
Department of Chemical Engineering
810 Mudd bldg, MC4721
500 West 120 th st.
New York , NY 10027
Phone: 212-854-3120
FAX: 212-854-3054
Email: jk1191@columbia.edu
Education:
B.S. 1974 University of Wisconsin, Chemical Engineering
Ph.D. 1979 University of Massachusetts, Chemical Engineering
1980 Centre de Recherches sur les Macromolecules, Strasbourg, France, NSF--CNRS and NATO Postdoctoral Fellow
Experience:
| 1980-86 | Assistant Professor of Chemical Engineering | Princeton University |
| 1981 | Visiting Assistant Professor | University of Wisconsin |
| 1985 | Visiting Research Scientist, IBM Research Labs | San Jose, California |
| 1986-89 | Associate Professor Chemical Engineering | University of Connecticut |
| 1989-92 | Founding Director, Polymer Compatibilization Research Consortium | |
| 1989-99 | Professor of Chemical Engineering | University of Connecticut |
| 1993-94 | Guest Professor, Max-Plank Institut | Mainz, Germany |
| 1994-98 | Director, Polymer Science Program | University of Connecticut |
| 1994-99 | Director, NSF Graduate Traineeship Program in Environmental Aspects of Plastics Recycling | University of Connecticut |
| 1996-99 | Co-Director, Biomaterials Design Initiative | University of Connecticut |
| 1998-99 | Distinguished Professor of Engineering | University of Connecticut |
| 2000-05 | Professor and Chair, Dept. of Chemical Engineering | Columbia University |
| 2002- | Professor in Residence | Columbia University |
| 2002- | Co-Director, NSF IGERT grant on Soft Materials | Columbia University |
| 2003- | Percy and Vida Hudson Professor of Chemical Engineering | Columbia Univ. |
| 2004 | Japan Society for the Promotion of Science Fellowship | Kyushu University, Japan |
Publications/Presentations:
Refereed Journal/Book Articles 109
Conference Presentations 248 (108 invited)
Invited Lectures/Seminars 241
Honors:
Arthur K. Doolittle Award, Amer. Chem. Soc., Div. Polym. Sci. Eng., 1984
ANTEC Best Paper Award, Society of Plastics Engineers, Plast. Anal. Div., 1985
American Cyanamid Academic Award, 1990
Elected Fellow, American Physical Society, 1992
Elected Member, Connecticut Academy of Arts and Sciences, 1994
Rogers Teaching Award, Department of Chemical Engineering, 1998
Distinguished Engineering Professorship, School of Engineering, 1998
Percy and Vida Hudson Chair in Chemical Engineering, 2003
Stine Award, American Institute of Chemical Engineers, 2006
Professional Society Memberships and Offices Held:
American Institute of Chemical Engineers, Material Engineering and Sciences Division
2 nd-Vice Chair: 2004
1 st Vice-chair: 2005
Program Chair: 2005
Chair: 2006
American Physical Society, Division of High Polymer Physics
Publication Comm.: Chair-1986
Program Comm.: 1989, 1990-Chair
Education Committee: 1994-Chair
Padden Award Committee: 1994-Chair
Ford Prize Committee: 1995, 1996-Chair
Executive Committee: Vice-Chair of Division-1994
Chair Elect of Division-1995
Chair of Division-1996
Past Chair of Division-1997
American Chemical Society, Polymer Division
North American Thermal Analysis Society
Awards Committee: 1987
Society of Plastics Engineers, Engineering Properties and Structure Division
Board of Directors: 1989-1990
Materials Research Society
Adhesion Society
Program Chair: 2003
Gordon Conference on Adhesion
Vice-Chair: 2006
Chair: 2008
Ongoing Research Projects:
1) Self-Assembling Photoactive Polymer Surfaces:
There is much current interest in developing polymer surfaces that are adaptive and responsive to various stimuli. The goal of this research is to create surface-active polymers that deliver various photoactive functional groups and polymers to the surface. Several recent publications are exemplary in demonstrating how this concept can be employed to create interesting surfaces. The first example is a surface that is initially hydrophobic and becomes hydrophilic when exposed to UV radiation. To accomplish this, diblock copolymers with a poly(t-butyl acrylate) block [PtBA] are used to modify the surface of a polymeric substrate. These copolymers form a bilayer structure at the polymer surface by surface segregation to present a surface layer of hydrophobic, low surface tension PtBA. The PtBA is transformed to hydrophilic polyacrylic acid (PAA) upon exposure to VU radiation in the presence of a photoacid generator by means of photocatalyzed hydrolysis. ["Photochemical Modification and Patterning of Polymer Surfaces by Surface Adsorption of Photoactive Block Copolymers" F. Pan, P. Wang, K. Lee, A. Wu, N. J. Turro, J. T. Koberstein, Langmuir, 21, 3605-3612, 2005.]

A 150 micron periodic pattern of carboxylic acid groups on a poly(tert-butyl acrylate) background imaged by water condensation.
When a single t-butyl acrylate group is placed at the end of a self-assembling monolayer (SAM), a similar approach can be used to create an atomic layer of carboxylic acid groups on the surface of glass or metallic (e.g., gold) substrates. [ “Photolithographic Technique for Direct Photochemical Modification and Chemical Micropatterning of Surfaces”, K. Lee, F. Peng, G. T. Carroll, N. J. Turro and J. T. Koberstein, Langmuir, 20, 1812-1818, 2004.]
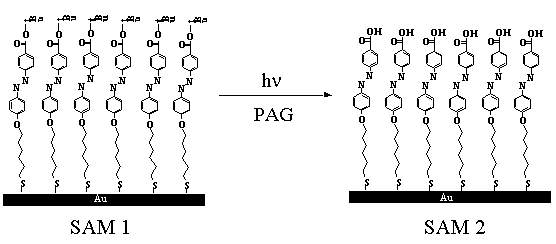
Photochemical creation of surface carboxylic acid groups on self-assembled monolayers.
2) DNA and Carbohydrate Microarrays.
Because both methods are activated by light, these concepts can be employed to create patterns of functional groups on surface which can allow for spatially directed reactivity, adsorption and self-assembly on virtually any substrate material. We have used these methods to control surface wetting patterns as well as to spatially pattern the covalent attachment of biologically important macromolecules such as proteins (e.g. antibodies), peptides e.g., cell adhesion ligands), DNA and carbohydrates (e.g., antigens). The latter systems are important platforms for the creation of diagnostic arrays and biosensors.
One of the most recent applications of photoactive surface technology is the formation of self-assembled monolayers that are capable of photografting a polymer to the surface by exposure to UV radiation. This new technique enables a straightforward means to create microarrays of carbohydrates as antibody sensors either by spotting or by direct photopatterning. [“Photochemical Micro-patterning of Carbohydrates on a Surface”, G. T. Carroll, D. Wang, N. J. Turro and J. T. Koberstein, Langmuir, in press]
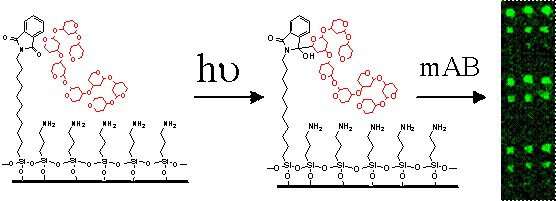
Covalent photoimmobilization of sugars onto modified glass slides and development by incubation with fluorescently tagged antibodies.
3) Surface Characterization and Modification of Nanoparticles:
Much of the excitement surrounding the topic of nanotechnology is associated with the fact that many properties of nanoparticulate matter are dependent upon particle size when the particle diameter is of the order of several nanometers: the emission wavelength of quantum dots and photonic crystals can be tuned by changing nanoparticle diameter, magnetic nanoparticles exhibit monodomain morphology and superparamagnetism within a certain range of particle diameter, and the human body reacts to nanoparticles in distinctly different manners depending upon the particle diameter and chemical nature of their surface.
While the intrinsic properties of nanoparticle systems are dependant primarily on their size, their applicability, in terms of commercialization, is primarily dictated by surface ligands that control their synthesis, solubility, self-organization, controlled placement/delivery to a specified site, and other properties. The ligand of choice for the synthesis of many metal oxide nanoparticle syntheses is oleic acid, yet there is no clear understanding of why this particular ligand seems to be optimal. The role of the ligand used for the synthesis is two-fold: first, it must limit the particle growth to the nanometer length scale and, second, it must stabilize the resultant nanoparticles against aggregation. The solution of nanoparticles, obtained after synthesis, has little direct applicability. In most cases, additional ligands must be added to ensure that the nanoparticles are well dispersed into some matrix or solvent, either by ligand exchange or direct ligand growth on the surface of the nanoparticle. Exchange of the oleic acid synthesis ligands on iron oxide nanoparticles with a-carboxylic acid functional poly(dimethyl siloxane) oligomers provides a means to control the interparticle distance in thin films made from this system.
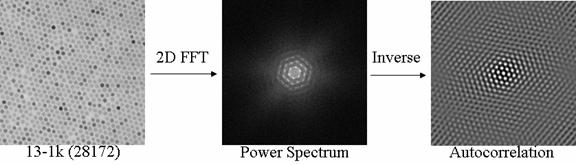
Transmission Electron Microscopy image of silicone modified naoparticles (left); power spect5um of the image (center) and autocorrelation function (right) used to determine the interparticle distance.
The second aspect of this research project is to develop novel and “universal” ligand chemistries for the synthesis and surface functionalization of nanoparticles; and to use these methods to synthesize iron oxide nanoparticles for a wide variety of biologically relevant applications. For this purpose, azide functionality is incorporated on the surface of the nanoparticles. Any biological ligands, including proteins, growth factors, peptides or DNA can be attached to these surfaces by incorporating alkyne functionality into the biomacromolecule.
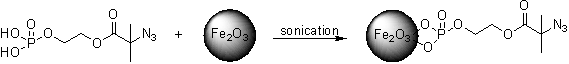
4) Model Polymer Networks and Hydrogels
Model polymer networks can be made from difunctional macromonomers, however there are few of such macromonomers available commercially. Difunctional macromonomers are synthesized using Atom Transfer Radical Polymerization (ATRP) to incorporate alkyne chain ends. [ “ATRP Synthesis and Characterization of a,w -allyl-terminated Macromonomers as Precursors for End-linked Gels and Hydrogels”, L. Voytova, N. J. Turro, J. T. Koberstein, Mat. Res. Soc. Symp. Proc. Vol. 774, 59-73, 2003.] When reacted with tri-functional azide compounds, a model end-linked polymer network is formed by Sharpless click chemistry, i.e., the reaction between and azid and an alkyne. During the macromonomer synthesis, a cleavable linkage is incorporated into the molecule that later can be cleaved using either ozone or light. In this fashion model networks can be formed but later destroyed by breaking the network at sites where there is a cleavable linkage. Such materials have a myriad of applications where it is desirable to first have a strong material, but then remove that material at a later time.
[“End-linked poly(tert-butyl acrylate) and poly(acrylic acid) networks from atom transfer radical polymerization and “click chemistry”, novel organo/hydrogels”,Jeremiah A. Johnson, David D. Díaz, M.G. Finn, Jeffrey T. Koberstein, Nicholas J. Turro, JACS, submitted]
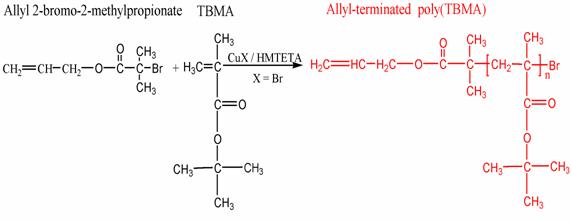
ATRP synthesis of a,w-functional Poly(tert-butylacrylate) macromonomer.
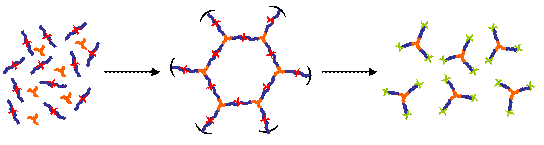
Alkyne-terninal macromomonomers (blue lines) containing cleavable groups (red X’s) are crosslinked with azide-tri-functional crosslinkers (orange three arm stars) by so-called “Sharpless click chemistry” to form model networks. These networks can then be cleaved by degradation of the cleavable groups.