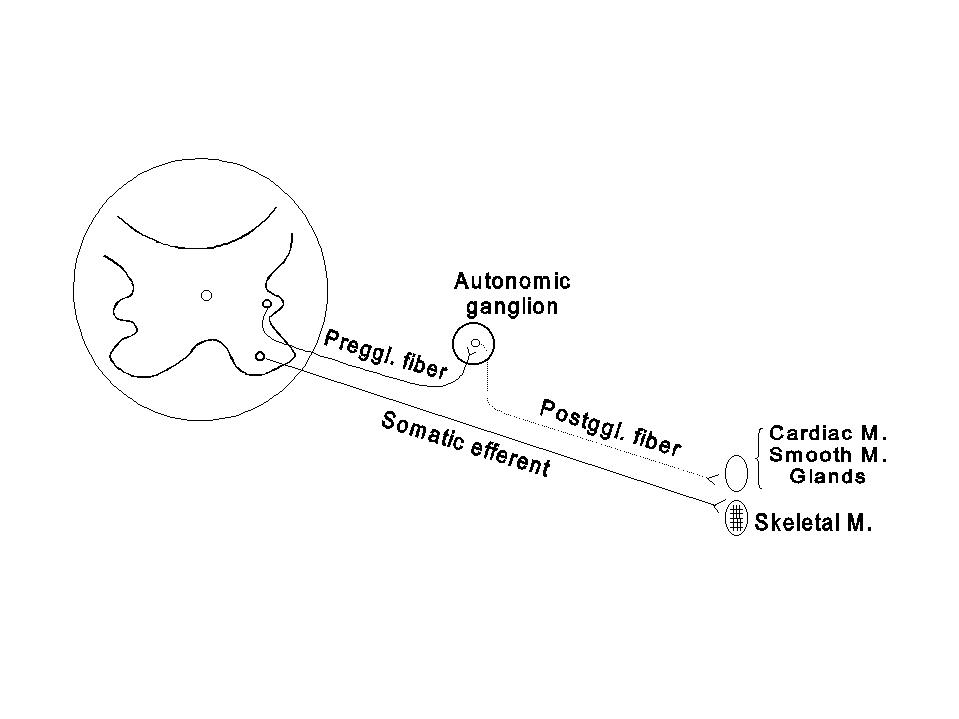
1.1 INTRODUCTION AND COMPARISON WITH SOMATIC EFFERENTS
The autonomic nervous system is comprised of the efferent neurons that innervate smooth muscle, cardiac muscle and the exocrine glands. Thus, it is distinguished from the somatic efferent nervous system, which supplies skeletal muscle. The afferent nerves in the visceral and somatic nervous system are similar in their structural and functional features, but the efferent nerves in these systems differ in many aspects, as in the following figure and table:

| Somatic efferents | Autonomic nerves | |
|---|---|---|
| 1. Structures innervated | Skeletal M. | Cardiac Muscle, Smooth Muscle, Glands |
| 2. Location of cell bodies | Entirely in CNS | Ganglia outside CNS |
| 3. Myelination | + | Preganglionic (+), Postganglionic (-) |
| 4. Neuroeffector junction | Endplates | Nerve plexuses, nodules |
| 5. Voluntary control | Yes | No |
| 6. Peripheral inhibition | No | Yes |
| 7. Effectors after denervation | Paralysis, atrophy | Automaticity, no atrophy |
| 8. General functions | Posture, movement | Homeostasis |
1.2 STRUCTURAL CONSIDERATIONS
The visceral efferent fibers originate from the autonomic neurons located in the lower brain stem and the spinal cord. These autonomic neurons are under the influences of (a) the incoming impulses from visceral or somatic afferent fibers, and (b) the descending impulses from the higher parts of the central nervous system, especially the limbic system and the hypothalamus.
The autonomic nervous system is classified into two sub-systems according to the level at which the nerves leave the central nervous system. The sympathetic nervous system is composed of the autonomic nerves exiting from the thoracic and lumbar segments of the spinal cord, whereas the parasympathetic nervous system consists of the nerves emerging from the lower brain stem and the sacral cord. Although the classification is made on an anatomical basis, many structural, functional and biochemical differences exist between these two systems (see table below). There are, however, also some similarities between the two systems.
| Structure or function | Sympathetic | Parasympathetic |
| I. Anatomical | ||
| A. Outflow from CNS | Thoracolumbar | Craniosacral |
| B. Location of ganglia | Close to CNS | Close to effectors |
| C. No. pre-/post-ggl. neurons | More post- | Ratio close to 1 |
| D. Distribution | Throughout the body | More limited |
| II. Functional | ||
| A. Cardiac muscle | ||
| 1. Heart rate | Increased | Decreased |
| 2. Force of vent. cont. | Increased | Little effect |
| B. Smooth muscles | ||
| 1. Blood vessels | Gen. constricted* | Little effect† |
| 2. Iris | Pupils dilated | Pupils constricted |
| 3. Bronchi | Dilated | Constricted |
| 4. Gastrointestinal tract | Inhibited (usu.) | Stimulated (usu.) |
| 5. Urinary bladder | Inhibited | Contracted |
| 6. G.I. and Urin. Sphincters | Contracted (usu.) | Relaxed |
| 7. Uterus | Variable | Variable |
| 8. Pilomotor muscles | Contracted | - |
| 9. Spleen capsule | Contracted | - |
| C. Glands | ||
| 1. Salivary glands | Stim. (viscous) | Stim. (watery) |
| 2. Gastrointestinal glands | Inhibited (usu.) | Stimulated |
| 3. Sweat glands | Stimulated* | - |
| 4. Adrenal medulla | Stimulated* | - |
| D. Metabolism | ||
| 1. Liver | Glycogenolysis | - |
| 2. Adipose Tissue | FFA release | - |
| E. General homeostasis | Energy mobilization | Protection, conservation and |
| restoration of resources, excretion | III. Chemical | |
| A. Neuroeffector transmitter | Mostly NE* | Acetylcholine |
| B. Transmitter destruction | Slow | Rapid |
| C. Systemic reinforcement | Secretion of NE and | - |
| Epi. by adr. med. | ||
*In the cases of the innervations to some blood vessels in the skeletal muscle, to most sweat glands, and to the adrenal medulla, sympathetic neuroeffector transmission is mediated by acetylcholine rather than norepinephrine (NE).
†Some blood vessels in facial and pelvic regions are dilated by parasympathetic impulses.
A. THE SYMPATHETIC NERVOUS SYSTEM (Thoracolumbar Outflow)
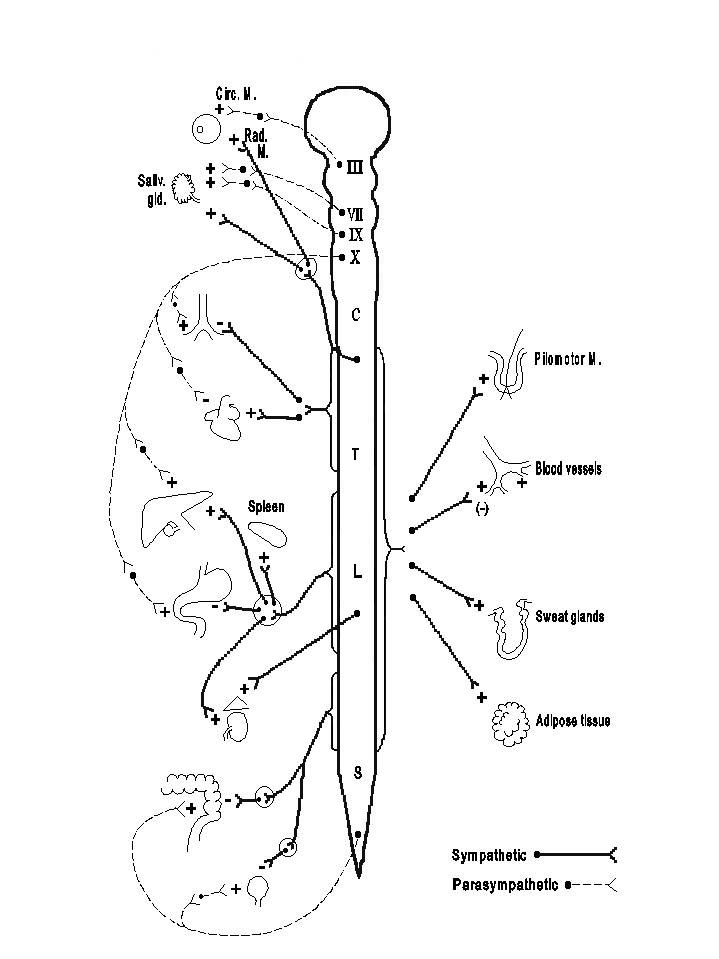
The cell bodies of the preganglionic neurons are located in the intermediolateral gray matter of the spinal cord from T1 to L2 or L3. The axons travel in the ventral roots and enter the spinal nerves at the corresponding levels. From the spinal nerves, these sympathetic preganglionic fibers leave as the white rami communicates to reach the vertebral (or paravertebral) sympathetic ganglion chains. Each of the bilaterally located chains contains one ganglion for each segment, corresponding to the level of exit of the preganglionic fibers. In addition, there are cervical ganglia (superior, middle, and inferior) and sacral ganglia, which receive the thoracolumbar preganglionic fibers turning upward and downward, respectively, in the sympathetic chains. The preganglionic fibers emerging from the thoracolumbar cord and going toward the vertebral chains terminate in one of the following ways: (1) they may terminate in the vertebral ganglia at the same level; (2) they may go through the vertebral ganglia and turn up or down in the vertebral chains to make synapses with the ganglion cells at the higher or lower levels; (3) they may go through the vertebral ganglia and terminate in one of the prevertebral ganglia (coeliac, superior mesenteric, and inferior mesenteric) or the terminal ganglia found in the wall of the urinary bladder and the rectum*), which are more distal from the spinal cord than the vertebral ganglia; or (4) the preganglionic fibers leaving T8 - T12 may go through the vertebral ganglia and terminate directly at the adrenal medulla without any postganglionic neuron. Each sympathetic preganglionic fiber branches to make synapses with many ganglionic neurons (divergence), and each ganglion cell may receive several preganglionic fibers (convergence). The degree of divergence is usually greater than that of convergence; e.g., in the superior cervical ganglion the ratio of pre- to postganglionic fibers is approximately 1 to 30. Because they branch significantly and travel in many different directions after reaching the vertebral chains, the sympathetic preganglionic fibers emerging from one spinal segment can give rise to postganglionic fibers innervating effectors distributed in many parts of the body. Thus, generally speaking, the sympathetic nervous system is organized for diffuse activities. The effector organs receiving sympathetic innervation are shown in the Simplified Diagram of the Autonomic Nervous System, on the next page.
*The ganglia and postganglionic fibers of the sympathetic and parasympathetic systems together with the non-adrenergic non-cholinergic fibers in the intestine form local nerve plexuses in the wall, and are referred to as the enteric nervous system.
B. THE PARASYMPATHETIC NERVOUS SYSTEM (Craniosacral Outflow)
The parasympathetic nervous system is generally organized for discrete and localized activities. The cell bodies giving rise to the cranial outflow are located in the brain stem nuclei of cranial nerves III (oculomotor N.), VII (facial N.), IX (glossopharyngeal N.), and X (vagus N.), and the preganglionic fibers travel in these corresponding cranial nerves. The cell bodies for the sacral outflow are found mostly in spinal segments S2 to S4. The preganglonic fibers emerge with the corresponding ventral roots and then leave the somatic efferent fibers to form the pelvic nerves. The parasympathetic preganglonic fibers terminate in ganglia situated in or near the organ innervated (terminal ganglia), and there is a lack of interconnection between ganglia comparable to the sympathetic vertebral chain. Parasympathetic fibers generally have a limited distribution to innervated organs, and only the vagus nerve supplies a large number of visceral structures. The effector organs receiving parasympathetic innervation are shown in the Simplified Diagram of the Autonomic Nervous System.
1.3 FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM
A. THE SYMPATHETIC NERVOUS SYSTEM
The sympathetic nervous system is stimulated when an individual is exposed to stressful situations (e.g., hemorrhage, trauma, anesthesia, emotional excitement, hypoglycemia, hypoxia, cold or exercise). These stimuli act through afferent nerves, or directly on the central representation of the ganglionic neurons. The sympathetic impulses then modify the activities of the innervated effectors either by excitation or inhibition, as summarized in the table on Comparison of Sympathetic and Parasympathetic Nervous Systems. Sympathetic activities generally serve to mobilize the energy stores of the body, to increase the blood flow through certain regions (e.g., the heart) at the expense of other areas (e.g., the abdominal viscerae), and to exhibit the outward signs of alarm and excitement. Because the sympathetically innervated effectors generally have a wide distribution, even when the sympathetic supply to only one type of effector (e.g., the vasomotor fibers to the cutaneous blood vessels) is stimulated, the bodily response is diffuse. The sympathetic innervation to certain parts of the body is tonically active in normal life, but the sympathetic nervous system is not essential for survival in a protected environment. When a sympathectomized animal is exposed to the various froms of stress which normally elicit or increase sympathetic activities, however, its deficiency becomes manifest.
B. THE PARASYMPATHETIC NERVOUS SYSTEM
The individual parasympathetic nerves are usually activated separately. When each parasympathetic nerve is stimulated, the responses as listed in the Table on Comparison of Sympathetic and Parasympathetic Nervous Systems can be obtained. The parasympathetic system is primarily concerned with protective functions, the restoration of bodily resources, the conservation of energy, and the elimination of waste products.
C. ANTAGONISM AND SYNERGISM OF THE SYMPATHETIC AND PARASYMPATHETIC NERVOUS SYSTEMS
Most effector organs receive dual innervation, but some (e.g., adrenal medulla, sweat glands, pilomotor muscles, and many blood vessels) are innervated by only the sympathetic nervous system. In those effectors receiving dual innervation, the actions of the two systems are usually opposite (see Table on Comparison of Sympathetic Nervous Systems), though not always so. Thus, the stimulation of either the sympathetic or the parasympathetic innervation results in salivary secretion. In cases where the actions of the dual innervation are antagonistic, the state of activity of the effector organ depends upon the balance of influences from the sympathetic and parasympathetic systems. The reflex organization is such that generally, when one system increases its rate of discharge, the other decreases its activity. Thus, as far as the effector is concerned, the opposite changes of the dual innervation actually represent a concerted effort resulting in the same response. For example, after hemorrhage, there is an increase in sympathetic discharge and decrease in vagal discharge, both leading to an increase in heart rate. Both divisions of the autonomic nervous systems play roles in the maintanence of homeostasis. The parasympathetic system is generally predominant in resting conditions, whereas the sympathetic system is usually activated in response to emergency situations.
1.4 JUNCTIONAL TRANSMISSION IN THE AUTONOMIC NERVOUS SYSTEM
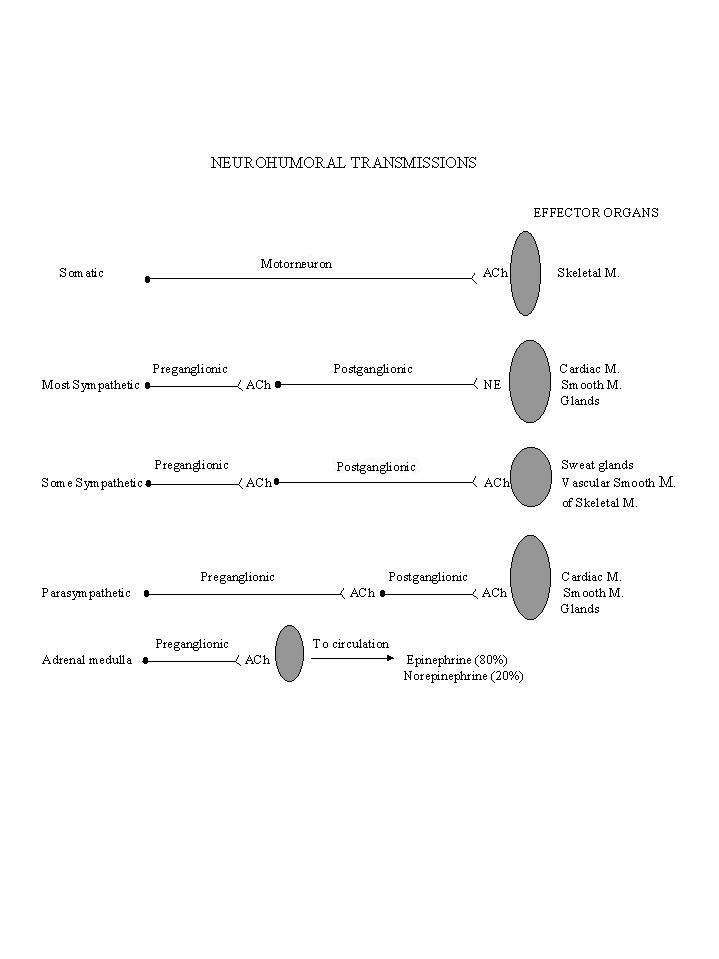
A. THE CONCEPT OF NEUROHUMORAL TRANSMISSION
The classical work of Otto Loewi demonstrated in 1921 that the transmission of activity from the vagus nerve to the heart is mediated through the liberation of chemical substance. Since then, it has been established that the transmission of nerve impulses across the autonomic ganglia (ganglionic transmission) and the transmission of activity from the autonomic nerves to the effector organs (neuroeffector transmission) are also accomplished by the liberation of certain chemical substances. The mediator for ganglionic transmission is acetylcholine in both the sympathetic and the parasympathetic system and in a few instances of the sympathetic system (e.g., the innervation to the sweat glands, to some of the blood vessels supplying the skeletal muscles, and to the adrenal medulla) neuroeffector transmission is also mediated by the release of acetylcholine. The neuroeffector transmission in the adrenal medulla, which is derived embryologically from the same source as the sympathetic ganglia innervated directly by preganglionic fibers, is comparable to that in the ganglia. The neuroeffector transmission in most of the sympathetic nervous system, except these just mentioned above, is mediated by norepinephrine. These sympathetic post-ganglionic fibers are called adrenergic, in contrast to all the other fibers in the autonomic nervous system which release acetylcholine and are termed cholinergic.
B. CHOLINERGIC MECHANISMS
(1) Metabolic turnover of acetylcholine.
The biosynthesis, storage and release of acetylcholine by the cholinergic fibers in the autonomic nervous system are essentially the same as in the somatic efferents. The nodular enlargements of the autonomic cholinergic nerve terminal contain a large number of synaptic vesicles (aproximately 30 nm in diameter), in which acetylcholine is stored in combination with a storage protein (see Diagram on next page). The spontaneous, random release of the vesicular content into the junctional cleft results in the combination of acetylcholine with a small percentage of the postjunctional receptor sites and the generation of miniature postjunctional potentials. The arrival of impulses at the cholinergic terminals, by inducing an influx of Ca ++, causes the synchronous release of a large amount of acetylcholine, which combines with a sufficient number of postjunctional receptor sites to result in an excitation or inhibition of the postjunctional element. The termination of the action of acetylcholine on postjunctional elements is mainly due to hydrolysis by cholinesterase and partially due to diffusion away from the receptor sites.

(2) Actions of acetylcholine.
Acetylcholine released by the preganglionic nerve endings causes a depolarization of the ganglionic cell and the generation of action potentials which propagate down the postganglionic nerve fiber. The acetylcholine released by the postganglionic nerve endings may cause either excitation or inhibition of the effector organ. As shown in the Diagram on Peripheral Actions and Possible Mechanisms of Action of Acetylcholine, acetylcholine retards the spontaneous depolarization of the cardiac pacemaker fibers, causing a slowing of the heart rate; it depolarizes the intestinal smooth muscle fibers, leading to an increase in mechanical tension; and it hyperpolarizes the sublingual gland cell, promoting salivary secretion. These examples are given only to illustrate that the action of acetylcholine, as well as its mechanism of action, differs considerably in various effector organs.

(3) Specificity of cholinergic receptors
The action of acetylcholine on postjunctional elements can be mimicked by a number of chemical substances (cholinomimetic agents), and it can be blocked by others (cholinergic blocking agents). The affinity for cholinomimetic and cholinergic blocking agents is different between the receptor sites in the autonomic ganglia (ganglionic receptors or nicotinic receptors) and those in the autonomic effectors (gamma-receptors or muscarinic receptors), indicating a difference in the chemical structure or conformation of the receptor proteins in these sites (see Diagram on Cholinergic Mechanisms). Nicotine is a cholinomimetic agent acting specifically on the autonomic ganglia. Nicotinic (ganglionic) receptors are ion channels composed of five subunits which open when the channels bind with nicotine or acetylcholine. Muscarine, an alkaloid present in certain poisonous mushrooms, is an effective cholinomimetic agent on autonomic effectors but not on the ganglia. The muscarinic action is mediated by muscarinic (gamma) receptors that are of the seven-pass family. When the receptors bind with muscarine or acetylcholine, the activated receptors interact with G proteins to activate phospholipase C in smooth muscle and glands and open potassium channels in cardiac tissues. Since acetylcholine has high affinities for the receptor sites in both the autonomic ganglia and the effectors, it is said to posses both a nicotinic action (on the ganglia) and a muscarinic action (on the effectors).
Cholinergic blocking agents also have differential affinities between autonomic ganglia and effectors. Hexamethonium is an example of a ganglionic blocking agent which can block the ganglionic action of acetylcholine by competitive inhibition. On the other hand, atropine is a classical cholinergic blocking agent at the neuroeffector junction.
| Receptor Subtype | Signaling Pathway | Agonists | Antagonists |
| Nicotinic | Ion channel | ACh, Nicotine | Hexamethonium |
| Muscarine | IP3 and DAG (smooth muscle & gland) | ACh, Muscarine | Atropine |
| Opens K+ (cardiac tissue) | |||
C. ADRENERGIC MECHANISMS
(1) Metabolic turnover of catecholamines.
The transmitter in adrenergic nerves is predominantly, if not exclusively, norepinephrine (also called noradrenaline). The small amount of epinephrine (or adrenaline) usually detected in the analysis of adrenergic nerves probably has its origin in the associated chromaffin tissues or represents the epinephrine transported from the extracellular fluid into the adrenergic nerve terminal. The adrenal medulla contains and secretes both norepinephrine and epinephrine. Norepinephrine and epirephrine are catecholamines, each having catechol nucleus (dihydroxybenzene) and an amine end group.
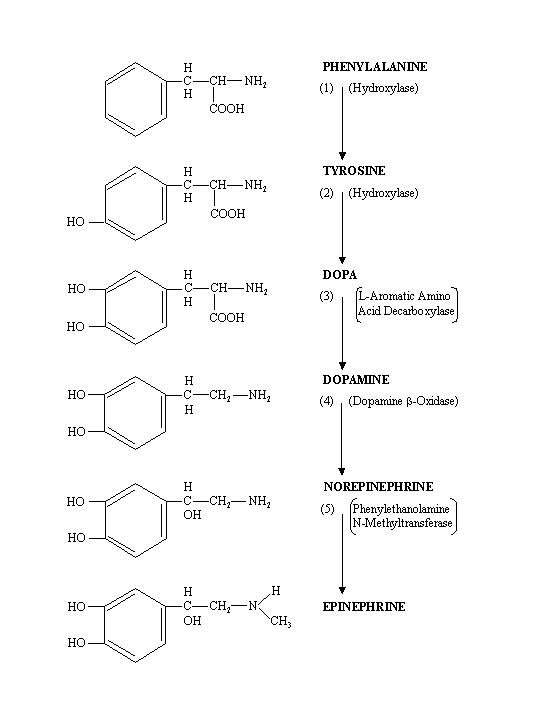
a. Biosynthesis of catecholamines.
The biosynthetic pathway is summarized in the following diagram. In the adrenergic nerves and in some adrenal medulla cells, norepinephrine represents the final product. In the other adrenal medulla cells, the presence of phenylethanolamine N-methyltransferase causes the conversion of norepinephrine into epinephrine. Dopamine is also a catecholamine and is secreted by the adrenal medulla. Its physiological action, however, is insignificant.
b. Storage of catecholamines.
The adrenergic nerve terminals have a series of nodular enlargements which contain vesicles approximately 100 nm in diameter. Most of the norepinephrine in the adrenergic nerve terminal is found inside the vesicles, mainly in a bound form. The adrenergic vesicles have a high concentration of ATP, with a norepinephrine: ATP molar ratio of 4:1. Therefore ATP may play a role in the binding and storage of norepinephrine inside the vesicle. The adrenergic vesicle also contains a number of proteins (chromogranins) with unknown functions. In the adrenal medulla, norepinephrine-secreting cells and epinephrine-secreting cells contain vesicles rich in these respective catecholamines, as well as ATP and chromogranins.

c. Release of catecholamines.
In the absence of action potentials, the adrenergic nerves spontaneously release small quanta of norepinephrine into the neuroeffector junction, and the combination of the norepinehrine with a small number of postjunctional receptor sites may lead to the generation of miniature postjunctional potentials. The arrival of the action potential at the adrenergic nerve terminals causes an influx of Ca++, wich leads to a synchronous release of a large amount of norepinephrine from many storage vesicles. As a result of the combination of norepinephrine with a large number of postjunctional receptor sites, sufficient postjunctional membrane changes will occur to cause the excitation or inhibition of the effector organ. The release of catecholamines by the adrenal medulla in response to acetylcholine is also mediated by Ca++ influx.
d. Enzymatic degradation of catecholamines.
As indicated in the following diagram, there exist two enzyme systems which can cause metabolic degradation of catecholamines. One system acts on the amine end group, and the major enzyme is monoamine oxidase (MAO). The deamination and oxidation of the side chain remove the physiological actions of catecholamines. The other system consists of catechol-O-methyl transferase (COMT), which acts on the catechol nucleus. The transfer of a methyl group to the oxygen on the catechol nucleus leads to the formation of normetanephrine and metanephrine from, respectively, norepinephrine and epinephrine. These O-methylated compounds possess very little physiological actions of catecholamines. The two enzyme systems may act in tandem, leading to the formation of 3-methoxy-4-hydroxymandelic acid (VMA, vanillylmandelic acid), which is the major metabolite of catecholamines found in the urine. Most body tissues and organs contain both COMT and MAO, and the released catecholamines are usually first acted upon by COMT and later by MAO. The adrenergic nerve terminals contain a high concentration of MAO, which prevents excessive accumulation of dopamine and norepinephrine, thus maintaining the intraneuronal homeostasis of these catecholamines.
e. Uptake of catecholamines by the adrenergic nerve terminals.
The action of norepinephrine on postjunctional elements is terminated not only by enzymatic degradation and the diffusion away from the receptor sites, but also by an uptake of norepinephrine into the adrenergic nerve terminals. The uptake mechanism is considered to be the major mechanism to terminate the action of norepinephrine at the adrenergic nerve terminals. The membrane of adrenergic nerve terminals can also transport epinephrine and other catecholamines from the extracellular fluid into the axoplasma, but with less efficacy than the uptake of norepinephrine. The uptake mechanism serves to replenish the transmitter store in the nerve terminals after norepinephrine release, thus economizing the synthesis of norepinephrine.
f. Summary of metabolic turnover of catecholamines.
The metabolic turnover of norepinephrine in the adrenergic neuroeffector junction is summarized in the following diagram.

(2) Specificity of adrenergic receptors.
Studies on the relative responsiveness of various organs to norepinephrine (NE), epinephrine (E) and another catecholamine, isoproterenol (Iso), have shown that there are at least two types of adrenergic receptors, referred to as α and β receptors, each of which has at least two subtypes. The α-receptors show greater affinity for E and NE than for Iso; whereas the β-receptors have affinity in the order of Iso›E›NE for smooth muscle, and Iso›E=NE for cardiac muscle. These two types of receptors can also be distinguished from each other by the specificity of adrenergic blocking agents (see table on Adrenergic Receptor Subtypes).
The α and β receptors are further divided into subclasess. Each of the specific receptor subtypes operates by one of the classic signaling pathways. The α adrenergic receptors on the effector organs are referred to as α 1 receptors. There are other α receptors, known as α 2 receptors on the adrenergic nerve terminals which respond to NE by inhibiting further NE release. The β adrenergic receptors on the effectors are subdivided into β1 and β2 receptors: β1 receptors are located in the cardiac muscle, the juxtaglomerular cells of the kidney and adipose tissue, whereas β2 receptors are located in smooth muscles, liver and skeletal muscle.
| Receptor | Signaling Pathway | Effector Response | Agonists | Antagonists | Receptor |
|---|---|---|---|---|---|
| Subtype | Affinity | ||||
| Production of IP3 and DAG | Smooth muscle contraction; gland secretation | Phenylephrine | Prazosine | E≥NE››Iso | |
| Inhibition of adenylyl cyclase | Inhibition of NE release | Clonidine | Yohimbine | E≥NE››Iso | |
| Stimulation of adenylyl cyclase | Cardiac excitation | Dobutamine | Metoprolol | Iso›E=NE | |
| Stimulation of adenylyl cyclase | Smooth muscle relaxation | Terbutaline | Iso›E›NE | ||
(3) Actions of catecholamines.
Since norepinephrine is the adrenergic nerve transmitter, the effects of norepinephrine administration on various organs are closely similar to those of adrenergic nerve stimulation. Just as the case of acetylcholine, norepinephrine may exert different effects on the postjunctional membranes of different organs, leading to either excitation or inhibition.
Epinephrine has many actions similar to those of norepinephrine, but there are some differences, especially in the relative effectiveness of these two catecholamines on various organs. The differences in the actions of norepinephrine and epinephrine can be explained by the variations in the regional distribution of α- and β-receptors (see the following Table) and the differential affinity of the two catecholamines for these receptors.
α RECEPTORS |
β RECEPTORS | |
| CARDIAC MUSCLE | SA node* (+) β1 | |
| Atria* (+) β1 | ||
| Ventricles* (+) β1 | ||
| SMOOTH MUSCLE | Most blood vessels (+) α1 | Coronary and skeletal muscle vessels* (-) β2 |
| Radial m. of iris (+) α1 | Bronchial muscle (-) β2 | |
| G-I sphincters (+) α1 | Gastrointestinal muscle* (-) β2 | |
| Trigone & sphincter of urinary bladder (+) α1 | Detrusor of urin. bladder (-) β2 | |
| Pilomotor muscle (+) α1 | ||
| Spleen capsule (+) α1 | ||
| EXOCRINE GLANDS | Salivary glands (+) α1 | |
| Palm sweat glands (+) α1 | ||
| METABOLISM | Liver* (+) β2 | |
| Skeletal muscle (+) β2 | ||
| Adipose tissue* (+) β1 | ||
| OTHERS | Adrenergic nerve | Juxtaglomerular cells (+) β1 |
| terminal (-) α2 | ||
| Pancreatic islets (-) α2 | ||
| Platelets (+) α2 | ||
Although the distinction between α- and β-receptors is based on their relative affinity for adrenergic agents, such classification is useful in sorting out the mechanical responses of the smooth muscles to catecholamines. Adrenomimetic agents generally cause the smooth muscles containing α-receptors to contract and those containing β-receptors to relax. Such a generalization, however, does not apply to the cardiac muscle. Although the cardiac muscle contains β-receptors, it is stimulated rather than inhibited by adrenomimetic agents. The reactivity of the adrenergic receptors in the cardiovascular system to catecholamines will be treated in detail in the cardiovascular section.
D. DENERVATION SUPERSENSITIVITY
After the removal of the nerve supply, the denervated effector organ becomes supersensitive to the administered natural transmitter agents and many other substances. Thus, in order to elicit a given response, the chronically denervated effector requires much less of the transmitter agent than the normal. Such denervation supersensitivity is seen in the autonomic effectors innervated by both adrenergic and cholinergic fibers, in the ganglion cells, and also in the skeletal muscle. It seems that the responsible factor for the denervation supersensitivity is the lack of exposure of the receptor sites on the effector organs to the transmitter. In the skeletal muscle after denervation, there is an increase in the membrane area sensitive to the transmitter acetylcholine. It is possible that a similar situation exists in the autonomic effectors after denervation.