Chapter 5: REGIONAL CIRCULATIONS
As indicated previously, the arterial pressure is determined by the cardiac output
and the total peripheral resistance. The total peripheral resistance is the total
resistance in the entire systemic circulation which consists of many parallel circuits
to major organs and tissues. The resistances in these individual circuits are not controlled
in the same manner, and these resistances govern the distribution of cardiac output from the
left ventricle into different regions of the body.
5.1 REGIONAL BLOOD FLOW AND OXYGEN CONSUMPTION
The following Table summarizes the normal blood flow and oxygen consumption in major organs
and tissues under resting conditions. The blood flow through the splanchnic circulation (25% of
cardiac output) can be subdivided into the hepatic arterial flow (7% of cardiac output) and the
mesenteric-portal circulation (18% of cardiac output). The "Other Organs" include all parts of
systemic circulations not specifically listed in the table, e.g., blood flow through the bones,
many endocrine glands, urogenital system, bronchial circulation, etc. Because of the differences
in sizes of the different organs, blood flow and oxygen consumption are also expressed in terms
of values per 100 gm weight.
The function of blood flow is to transport materials to
and from tissues. In many regions of the
body, the main transport function is to meet the local metabolic demands. In several other regions, however, the
primary purpose of the circulation is to provide a large flow rate for the
processing of the physicochemical constituents of the blood, and the blood flow
is in excess of that needed to meet the local metabolic demands. A calculation of the ratio of blood flow to
oxygen consumption ( B/
B/ O2) serves to distinguish these
two types of purposes. The kidneys
regulate the balance of water and solutes, the lungs regulate the blood gases,
and the skin regulates heat balance. In
these regions the ratio
O2) serves to distinguish these
two types of purposes. The kidneys
regulate the balance of water and solutes, the lungs regulate the blood gases,
and the skin regulates heat balance. In
these regions the ratio  B/
B/ O2 is in excess of 70
ml/ml. The values for the lungs are not
shown in the table, but here the large pulmonary blood flow (5,000 ml/min) is not
concerned with gas exchange not local metabolic transport, which is met by the
bronchial circulation. The blood flow
and oxygen consumption in a given region determine the difference in O2
content between the arterial and venous blood (ΔA-VO2):
O2 is in excess of 70
ml/ml. The values for the lungs are not
shown in the table, but here the large pulmonary blood flow (5,000 ml/min) is not
concerned with gas exchange not local metabolic transport, which is met by the
bronchial circulation. The blood flow
and oxygen consumption in a given region determine the difference in O2
content between the arterial and venous blood (ΔA-VO2):
 O2 =
O2 =  B (ΔA-VO2)
or ΔA-VO2 =
B (ΔA-VO2)
or ΔA-VO2 =  O2
/
O2
/ B
B
Therefore, the A-VO2 difference is inversely related to the  B/
B/
 O2 ratio. Thus, the high
O2 ratio. Thus, the high  B/
B/ O2
ratios in the renal and cutaneous circulations are associated with a low A-VO2 difference and hence a high
venous O2 content.
O2
ratios in the renal and cutaneous circulations are associated with a low A-VO2 difference and hence a high
venous O2 content.
In most other organs and tissues, the blood flow mainly serves to meet
the local metabolic demand. In these
regions the ratio  B/
B/ O2 is less than 35 ml/ml, and the
A-V O2 difference is higher than 3 cc/100 ml. The coronary circulation has
the lowest
O2 is less than 35 ml/ml, and the
A-V O2 difference is higher than 3 cc/100 ml. The coronary circulation has
the lowest  B/
B/ O2
ratio, the largest A-V O2 difference and the lowest venous O2
content. These indicate that, in comparison to other regions, the myocardium has the least blood flow in
relation to its metabolic requirement and that a large extraction of O2 from the
blood is necessary to meet even the resting metabolic needs. This probably reflects the continuous
activity of the cardiac muscle even under “resting” conditions. Whereas other organs can considerably
increase their O2 extraction to meet enhanced metabolic
requirements, this reserve is rather limited in the myocardium.
O2
ratio, the largest A-V O2 difference and the lowest venous O2
content. These indicate that, in comparison to other regions, the myocardium has the least blood flow in
relation to its metabolic requirement and that a large extraction of O2 from the
blood is necessary to meet even the resting metabolic needs. This probably reflects the continuous
activity of the cardiac muscle even under “resting” conditions. Whereas other organs can considerably
increase their O2 extraction to meet enhanced metabolic
requirements, this reserve is rather limited in the myocardium.
REGIONAL BLOOD FLOW AND OXIGEN CONSUMPTION AT REST
|
| |
Blood Flow |
Oxigen Consumption |
Blood Flow |
A-VO2 |
Venous O2 |
| |
% ml ml/min |
% cc cc/min |
O2 Cons. |
Content |
Content |
| |
C.O. min 100 gm |
Total min 100 gm |
(ml/cc) |
(ml/dl) |
(ml/dl) |
|
| 1. Coronary |
5 250 70 |
13 30 8.4 |
8 |
12.5 |
6.5 |
| 2. Cerebral |
15 750 50 |
22 50 3.3 |
15 |
6.7 |
12.3 |
| 3. Splanchnic |
25 1250 50 |
24 53 2.1 |
24 |
4.2 |
14.8 |
| 4. Renal |
22 1100 400 |
7 15 5.5 |
73 |
1.4 |
17.6 |
| 5. Cutaneous |
8 400 10 |
2 5 0.1 |
80 |
1.2 |
17.8 |
| 6. Muscular |
17 850 2 |
27 60 0.2 |
14 |
7.1 |
11.9 |
| 7. Other regions |
8 400 3 |
5 12 0.1 |
33 |
3.0 |
16.0 |
|
| Total Systemic Circ. |
100 5000 7 |
100 225 3.2 |
22 |
4.5 |
14.5 |
|
*These values are only round figures. They show considerable individual variation due to
size and other factors. The venous O2 contents are calculated by using an arterial
O2 content of 19 ml/dl.
Not shown in this table is the blood flow through the pulmonary circulation, which receives
100% of the cardiac output from the right ventricle, with pulmonary blood flow of approximately
200 ml/min/100 gm.
The values given in the table represent those obtained under resting conditions in a
comfortable environment, and they can be altered significantly under a variety
of conditions. For example, muscular exercise would increase markedly the blood flow and
oxygen consumption in the exercising muscle. Exposure to a warm environment would increase
the blood flow through the cutaneous circulation.
5.2 CONTROL OF VASCULAR HINDRANCE IN DIFFERENT REGIONS
The blood flow ( x through a given region x is determined by the
pressure drop from arterial inflow to venous outflow (Pa-Pv) and the resistance in the
region (Rx):
x through a given region x is determined by the
pressure drop from arterial inflow to venous outflow (Pa-Pv) and the resistance in the
region (Rx):
 x = (Pa-Pv) / Rx
x = (Pa-Pv) / Rx
Since the values of Pa-Pv as well as
the blood viscosity are essentially the same in most regions,  x
varies inversely with the vascular hindrance in each region. As discussed above, vascular
hindrance is controlled by neurohumoral influences and by autoregulatory processes. The
following table summarizes the type of adrenergic receptors (α or β) present in
different regions, the primary effect of epinephrine, the presence or absence of sympathetic
adrenergic and cholinergic innervation and the effect of stimulation, and autoregulatory
control by metabolic (increased CO2 and acidity or reduced O2) and
mechanical factors (increased transmural pressure).
x
varies inversely with the vascular hindrance in each region. As discussed above, vascular
hindrance is controlled by neurohumoral influences and by autoregulatory processes. The
following table summarizes the type of adrenergic receptors (α or β) present in
different regions, the primary effect of epinephrine, the presence or absence of sympathetic
adrenergic and cholinergic innervation and the effect of stimulation, and autoregulatory
control by metabolic (increased CO2 and acidity or reduced O2) and
mechanical factors (increased transmural pressure).
REGULATION OF REGIONAL CIRCULATIONS
|
|
Neurohumoral Influences
Adr. Epi. Symp. N.S.
recep. (prim.) NE ACh |
Autorregulation
Metabolic Mechanical
↑CO2, ↓pH ↓O2 (↑P) |
|
| A. Systemic Circ. |
|
|
| 1. Coronary |
α , β D D - |
(D) D |
| 2. Cerebral |
- - - - |
D(CO2) (D) (C) |
| 3. Splanchnic |
|
|
| a. Hepatic art. |
α C C - |
|
| b. Mesent.-Port. |
α , β C C - |
D |
| 4. Renal |
α C C - |
C |
| 5. Cutaneous |
α , β C C - |
|
| 6. Muscular |
β*, (α) D C D |
D |
| B. Pulmonary Circ. |
(α) (C) (C) - |
C |
| |
|
(↓alv. pO2) |
|
*β receptors in blood vessels of skeletal muscle are not innervated
C: Constriction; D: Dilatation
A comparison of the mechanisms controlling vascular hindrance in different
regions indicate that vasoconstriction in response to sympathetic adrenergic
influence occurs primarily in the abdominal visceral organs (splanchnic region
and kidney), skeletal muscle and skin.The brain has no significant sympathetic
adrenergic influence, whereas the coronary circulation responds to sympathetic
adrenergic impulses by dilation. Therefore, reflex activation
of the sympathetic adrenergic system causes a redistribution of blood flow,
which is diverted away from the vasoconstricted areas and to the heart and the
brain.
Autoregulation by metabolic factors plays an important role in the control
of coronary blood flow (especially sensitive to hypoxia) and cerebral blood flow
(especially sensitive to changes in PCO2). Autoregulation by mechanical
factors also occurs in the cerebral circulation. Metabolic autoregulation is seen
in the mesenteric and muscular circulations, and the mechanical type of autoregulation
is observed in the renal circulation. Pulmonary circulation has limited sympathetic
adrenergic innervation. The pulmonary vasculature can respond to a local reduction in
alveolar PO2 by vaconconstriction, thus diverting pulmonary blood flow from
poorly ventilated areas to other areas that are better ventilated.
The factors regulating cerebral blood flow, renal blood flow (lectures on the
kydney) and pulmonary blood flow are be treated in greater detail elsewhere. In the
following two subsections the factors controlling coronary blood flow and blood flow
through the skeletal muscle will be discussed.
5.3 CORONARY CIRCULATION
The coronary supply to the heart is equal to approximately 5% of the cardiac output
under resting conditions. Of the total coronary flow approximately 85% is supplied by
the left coronary artery and approximately 15% is supplied by the right coronary artery.
The relative distribution of these two coronary arteries between the left and right
ventricles varies among individuals. The coronary circulation contains a rich capillary
network such that the number of capillaries is at least as large as the number of myocardial
fibers. The outflow from the left ventricle generally drains into the coronary sinus which
empties into the right atrium. The outflow from the right ventricular myocardium drains
primarily via the anterior cardiac veins, which empty into the right atrium. There are
also small amounts of venous blood collected by the Thebesian vessels that can
drain into either the right or left chambers. Normally the coronary sinus
outflow constitutes approximately 70% of the total venous drainage.
A. MECHANICAL FACTORS REGULATING CORONARY FLOW
The external pressure on the coronary circulation exerted by the force of contraction
of the myocardium has an important influence on coronary resistance, especially in the
left ventricular myocardium. During each phase of the cardiac cycle this extravascular
pressure changes, as does the aortic pressure. Since the coronary flow is determined by
the ratio of the driving aortic pressure to the coronary resistance, the phasic variations
in these two parameters result in a phasic change in coronary flow (see figure). During
diastole (0), when the ventricular myocardium is relaxed, coronary flow depends primarily
upon the aortic pressure. At the beginning of isovolumetric contraction (1), the extravascular
pressure increases very sharply, resulting in a marked rise in coronary resistance,
especially on the left side. Therefore, there is a marked decrease of the left coronary flow,
such that the flow is stopped or even reversed. Following the opening of the aortic valve (2)
the ejection of blood from the left ventricle reduces the extravascular pressure and coronary
resistance. Since the aortic pressure is rising at the same time, these changes cause a rise
in left coronary flow. During the period of reduced ejection (3), the coronary flow decreases
together with aortic pressure.
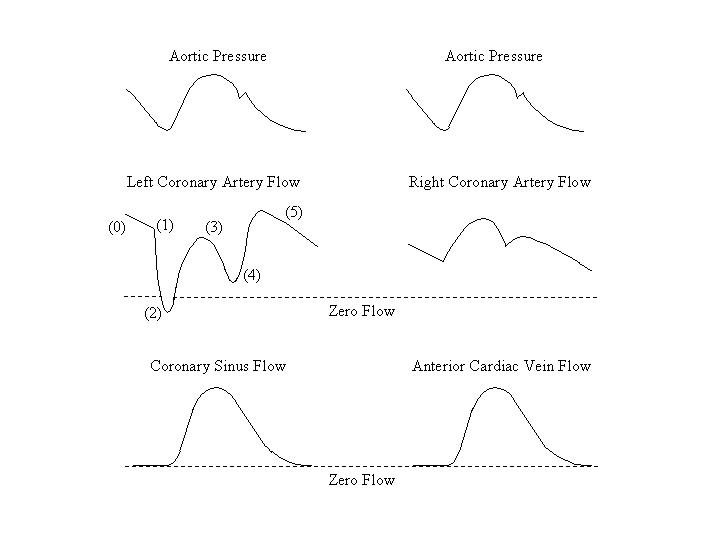
With the onset of isovolumetric relaxation (4), the extravascular pressure
decreases, and the resulting reduction in coronary resistance causes a rise in left
coronary flow. Thereafter (5), the coronary flow changes in the same direction as
the arterial pressure during diastole.
Because of the phasic nature of the left coronary arterial inflow and the relatively
longer duration of diastole than systole, approximately 3/4 of the left
coronary inflow occurs during diastole and only 1/4 during systole. Therefore,
excessive changes in the heart rate, by changing the relative duration of diastole
in the cardiac cycle, can affect left coronary flow.
The wall stress generated during ventricular contraction is the greatest on the
subendocardial surface, and it decreases progressively toward the epicardial
surface. Therefore, the compression of left coronary vessels is greater in the
subendocardium, leading to a vulnerability of subendocardial coronary blood flow.
Because the contraction of the right ventricle is considerably weaker than
that of the left ventricle, right coronary vessels are subject to much less
extravascular compression during systole. The phasic tracing of right coronary
blood flow follows more closely the contour of the aortic pressure tracing and
resembles the flow pattern in other regions of the body.
The variations in extravascular pressure in the coronary circulation also affect
the capacity of the veins and the volume of blood in the venous side of the coronary
circulation. During the contraction phase as the ventricular pressure increases, the size
of the veins is reduced and blood is squeezed out of the veins in the coronary circulation.
As relaxation begins and ventricular pressure drops, there follows an expansion of the
coronary veins and a reduction in venous outflow.
B. METABOLIC FACTORS
Oxigen. Under normal resting conditions the coronary circulation extracts a large
percentage of the oxygen delivered by the arterial supply (see Table on Blood Flow and Oxygen
Consumption at Rest). A reduction in coronary flow tends to cause a corresponding reduction
in myocardial PO2, since the A-V oxygen difference cannot be increased much further.
Decreases in myocardial PO2 exert a strong vasodilation influence on the coronary
circulation, and this constitutes the major autoregulatory mechanism of coronary flow.
Carbon dioxide and pH. An increase in PCO2
or decrease in pH causes vasodilation, but the effect is less pronounced than
that produced by a lowering of PO2.
C. NEUROHUMORAL FACTORS
Both sympathetic stimulation and catecholamines cause coronary vasodilation.
They do it indirectly, however, since the direct effect of sympathetico-adrenal
stimulation is vasoconstriction due to the greater preponderance of α receptors
over β receptors in the coronary vessels. Sympathetic activation stimulates
myocardial contractility; it is the metabolic changes that result from this stimulation
and that cause the vasodilation via autoregulation.
Vagal stimulation has two conflicting effects on the coronary vessels. It causes
vasoconstriction via direct stimulation of the vessel’s smooth muscle, but vagal stimulation
also stimulates the coronary endothelium to release EDRF (Endothelium Derived Relaxing Factor)
which relaxes coronary smooth muscle cells. The net effect of vagal stimulation is a slight
vasodilation, but the effect probably has little physiological significance.
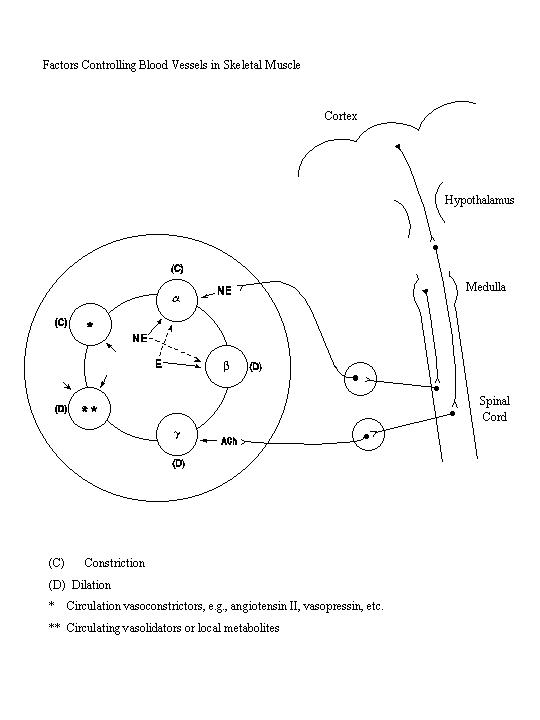
5.4 CIRCULATION THROUGH THE SKELETAL MUSCLE
The skeletal muscle comprises more than one half of the total body weight, but
it receives only approximately 17% of the cardiac output and uses only approximately
27% of the total oxygen consumed at rest. During muscular exercise, the vasodilation
and opening of previously non-circulating vessel cause marked increases in muscle
blood flow and oxygen consumption. The variations in blood flow and oxygen consumption
are much larger in the skeletal muscle than in other tissues and organs. Blood vessels
in the skeletal muscle are regulated by many different neural and humoral factors, and
their control is probably the most complicated among the various regional circulations
(see Figure).
A. SYMPATHETIC ADRENERGIC SYSTEM
The skeletal muscle vessels contain both α and β adrenergic receptors.
The α receptors are innervated by sympathetic adrenergic nerves. The activation
of sympathetic adrenergic system following hemorrhage, hypoxemia, or exposure to cold
causes the release of norepinephrine, which combines with the α receptors to
constrict skeletal muscle vessels. The β receptors in the skeletal muscle vessels
are more abundant than the α receptors. The β receptors in the skeletal muscle
are not innervated, however, and therefore respond only to the blood-borne catecholamines
secreted by the adrenal medulla or administered therapeutically. When catecholamines bind
to the β receptors of skeletal muscle vessels, the vessels dilate. Due to the greater
abundance of β receptors, the blood borne catecholamines, especially epinephrine,
cause vasodilation in the skeletal muscle.
B. SYMPATHETIC CHOLINERGIC SYSTEM
In addition
to the α and β adrenergic receptors, skeletal muscle vessels also contain
the γ cholinergic receptors which are innervated by the sympathetic cholinergic
nerves. As shown in the following diagram, the sympathetic cholinergic nerves are under
the influence of the cerebral cortex and the hypothalamus. The combination of the
acetylcholine released at the postganglionic nerve endings with the γ receptors
causes vasodilation of the skeletal muscle. This system is activated by anticipation of
exercise and by emotional stimuli, e.g., the sight of blood, and the resulting sudden
vasodilation in the skeletal muscle may cause significant reductions in arterial pressure
and cerebral blood flow, leading to fainting.
C. LOCAL METABOLIC ENVIRONMENT
The accumulation of CO2 and acid metabolites and the reduction in
PO2 cause vasoldilation in the skeletal muscle. This is the most
important factor causing muscular vasodilation during exercise, leading to
increases in skeletal muscle blood flow by a much as 20-25 fold and increases
in oxygen consumption of more than 40 fold.
 B/
B/ O2) serves to distinguish these
two types of purposes. The kidneys
regulate the balance of water and solutes, the lungs regulate the blood gases,
and the skin regulates heat balance. In
these regions the ratio
O2) serves to distinguish these
two types of purposes. The kidneys
regulate the balance of water and solutes, the lungs regulate the blood gases,
and the skin regulates heat balance. In
these regions the ratio  B/
B/ O2 is in excess of 70
ml/ml. The values for the lungs are not
shown in the table, but here the large pulmonary blood flow (5,000 ml/min) is not
concerned with gas exchange not local metabolic transport, which is met by the
bronchial circulation. The blood flow
and oxygen consumption in a given region determine the difference in O2
content between the arterial and venous blood (ΔA-VO2):
O2 is in excess of 70
ml/ml. The values for the lungs are not
shown in the table, but here the large pulmonary blood flow (5,000 ml/min) is not
concerned with gas exchange not local metabolic transport, which is met by the
bronchial circulation. The blood flow
and oxygen consumption in a given region determine the difference in O2
content between the arterial and venous blood (ΔA-VO2):