The basic building blocks for the world around us are the chemical elements. Some familiar elements include oxygen (chemical symbol: O) and hydrogen (H). Individual atoms of the chemical elements are composed of protons and neutrons in an atomic nucleus surrounded by electrons in their orbitals. Atoms of each element have a specific number of protons and an equal number of electrons. For example, all oxygen atoms contain eight protons and eight electrons. The number of electrons determines the chemical character and bonding behavior (what other elements it is likely to bond with, the strength of those bonds, etc.). When two or more atoms bond together they form a molecule. The air that we breath contains O2, which is a molecule made of two atoms of oxygen bound together. If there is more than one kind of atom in a molecule it is called a chemical compound. When we turn on the kitchen faucet, we fill our glass with the chemical compound H2O, in which each molecule is made of two atoms of hydrogen and one atom of oxygen. Minerals are one class of chemical compounds. There are several types of bonds between atoms. For our purpose we will only identify two: covalent bonds result from the sharing of electrons by two adjacent atoms; ionic bonds resulting when one atom completely gives up one or more electrons to an adjacent atom. In the weathering environment at the Earth's surface covalent bonds are generally strong and ionic bonds weak.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A mineral is a naturally occurring, inorganic, homogeneous solid with a definite (though variable) chemical composition and an ordered atomic arrangement.
A crystal is a regular geometric solid bounded by smooth plane surfaces (maybe a mineral crystal but there are also organic crystalline materials). The regular geometry is due to the ordered atomic arrangement. The angles between the faces of a free-grown crystal are controlled by the regular geometry of the atoms.
A rock is an assemblage of mineral grains.....
Mineral Classification
Minerals may be classified according to their chemistry. Some of the important mineral classes are listed below.
|
Mineral Class |
Basis |
Examples |
|
native elements |
uncombined elements |
gold (Au), diamond (C) |
|
oxides |
simple oxygen (O) compounds |
iron oxides-hematite (Fe2O3) |
|
|
|
-magnetite (Fe3O4) |
|
halides |
simple compounds with chlorine |
table salt-sodium chloride (NaCl) |
|
|
(Cl), fluorine (F), etc |
fluorite-calcium fluoride (CaF2) |
|
sulfides |
simple sulfur (S) compounds |
pyrite-iron sulfide (FeS2) |
|
|
|
galena-lead sulfide (PbS) |
|
carbonates |
compounds based on (CO3)-2 |
calcite-calcium carbonate (CaCO3) |
|
sulfates |
compounds based on SO4 |
gypsum-calcium sulfate |
|
silicates |
compounds based on the silica |
quartz-silicon dioxide (SiO2) |
|
|
tetrahedron structure |
potassium feldspar (KAlSi3O8) |
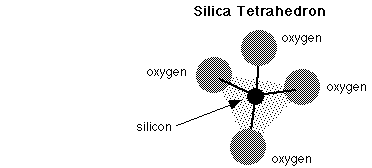
The silicate minerals are the most important mineral class because they are by far the most abundant rock-forming minerals. This group is based on the silica (SiO4) tetrahedron structure, in which a silicon atom is covalently bonded to 4 oxygen atoms at the corners of a triangular pyramid shape.

Tetrahedra may be isolated from one another or they may be bonded together covalently by sharing oxygen atoms between adjacent tetrahedra. In this way they may form single chains, double chains, sheets, and 3-dimensional networks of interlocking tetrahedra.
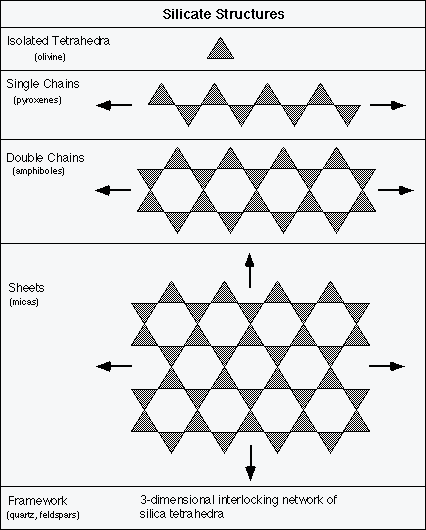
Each of these covalently bonded structural groups (except framework) is bonded to its neighboring structural group (e.g. single chain to single chain) by ionic bonds with the intervening cations (the K+, Na+, Ca2+, Mg2+, Fe2+, etc.). Cleavage (breaking along a planar surface) in the silicates forms along the planes of ionic bonds. For example, the sheet silicates (e.g., micas) cleave along the plane of ionic bonds that holds the adjacent tetrahedral sheets together. In framework silicates all tetrahedra are completely interlocked into a 3-dimensional structure. Quartz, which is composed of nothing but the covalently bonded silicon and oxygen, has no preferred cleavage planes. That is why quartz only displays a conchoidal fracture (circular, glass-like breakage) with no cleavage.
The most important rock-forming minerals are quartz, feldspars, micas, amphiboles, pyroxenes, and olivine.
Quartz, a framework silicate, is identified by its hardness (7), lack of cleavage, conchoidal fracture, and six-sided crystals. Color may be clear, pink, purple, milky white, gray, black, etc.
Feldspars, also framework silicates, are
separated into potassium feldspars (alkali feldspar) and
plagioclase feldspars. All feldspars are nearly as hard as
quartz (6) but have two cleavages at nearly right angles. One
cleavage direction is better than the other.
Potassium feldspars range from pink to white to gray (the variety
called amazonite is green). They often display a subtle pattern of
wavy stripes known as perthite
Plagioclase feldspars are white to gray to black (the labradorite
variety is iridescent blue) and usually show closely spaced
striations on the perfect cleavage plane due to alternating
twinned crystals.
Micas, sheet silicates, are easily identified by their one perfect cleavage and foliated (sheeted) habit. The dark, often black, variety is biotite. The transparent variety is muscovite. A third common variety, chlorite, is green and its habit tends toward small scales, rather than the large sheets of biotite and muscovite.
Clay minerals are also important sheet silicates. Their crystals are sub-microscopic. On a macroscopic scale they form earthy masses. They form as weathering products of other silicate minerals.
Amphiboles (e.g., hornblende) are double chain silicates and pyroxenes (e.g., augite) are single chain silicates. Amphiboles and pyroxenes are common dark minerals found in intermediate to mafic rocks. Each has 2 good cleavages. They can be differentiated by the angle between the cleavages. Amphiboles form long prismatic 6-sided crystals and the angles between the cleavages are 56&Mac251; and 124&Mac251;. hornblende is more lustrous than augite and has a splintery cleavage. Pyroxenes form stubby prismatic crystals and the angles between cleavages are about 90&Mac251;.
Olivine, an isolated tetrahedra silicate, is normally found as masses of olive green to brown granules. Each sand-sized olivine crystal has conchoidal fracture.
Calcite is a carbonate mineral (calcium carbonate). Calcite and related carbonates including dolomite (calcium magnesium carbonate) are the primary minerals in limestone which is a common sedimentary rock found in the upper crust of the Earth. Calcite has three perfect cleavages which do not meet at 90&Mac251;. Its hardness is 3. It dissolves under acidic conditions; it fizzes when dilute hydrochloric acid is dropped on it. Dolomite fizzes only very weakly.