|
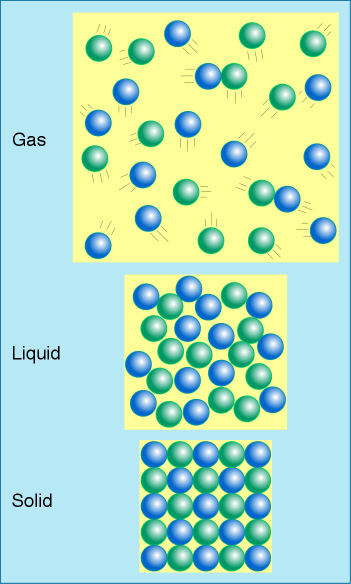
In the gaseous state, molecules have so much kinetic energy that they fly off in all directions but repeatedly collide and bounce off of other molecules. |
 |
|
--- boiling temperature - condensation temperature ---
|
|
|
In the liquid state, atoms or molecules have sufficient kinetic energy to overcome the chemical bonds that held them in their crystal lattice and move independently, yet they don't have enough energy to separate completely from other atoms.
|
|
|
--- melting temperature - crystallization temperature ---
|
|
|
In the solid state, chemical bonds are stronger than the kinetic energy of the atoms. The atoms are locked into their crystal lattice positions.
|