Stable Isotopes and Isotope Stratigraphy as Indicators of Changing Climate and Biosphere
Oxygen Isotopes
While most oxgen atoms have a mass of 16 (8 protons and 8 neutrons), a small number of oxygen atoms have a mass of 18 (8 protons and 10 neutrons). Both of these isotopes are stable; they do not undergo radioactive decay.
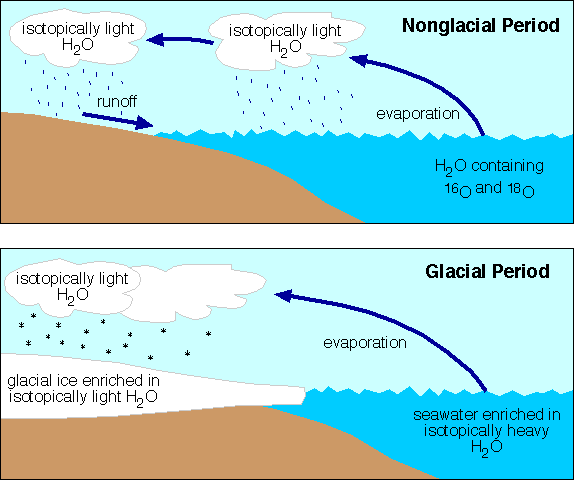
Water molecules (H2O) in the ocean may contain either isotope, oxygen 16 or oxygen 18. Water molecules containing oxygen 18 are heavier (18 atomic mass units vs. 20 atomic mass units since each hydrogen atom has a mass of just one).
Just as it is harder to throw a heavy object into the air than a light one, it is easier for water molecules containing the lighter oxygen 16 atoms to evaporate than the heavier oxygen 18 containing water molecules (the heavier ones need more kinetic energy to go into the gaseous state). In other words, as water evaporates from the ocean surface a disproportionately large amount of H2O-16 is evaporated compared to H2O -18. Therefore, the ratio of H2O-18 to H2O-16 in clouds formed from that evaporation is less ("lighter") than that in the seawater from which it evaporated.
If the moisture in those clouds falls as snow on a continent, and if that snow accumulates into an expanding continental ice sheet, then more and more isotopically light H2O will be stored on the continent and the remaining seawater will become progressively enriched in isotopically heavy H2O.
The oxygen isotopic ratio in carbonate shells is in equilibrium with that in seawater at the time that the shells form. Therefore, layer by layer study of the oxygen isotopic ratio in shells in marine sedimentary strata can yield a record of the waxing and waning of continental ice sheets. More oxygen 18 means more ice on the continents. More oxygen 16 means less ice.

Carbon Isotopes
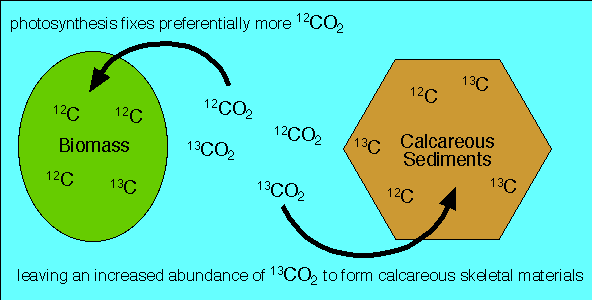
There are two stable carbon isotopes, carbon 12 (6 protons and 6 neutrons) and carbon 13 (6 protons and 7 neutrons). Photosynthetic organisms use disproportionately more CO2 containing the lighter carbon 12 than the heavier carbon 13 (the lighter molecules move faster and therefore diffuse more easily into plant cells where photosynthesis takes place).
During periods of high biological productivity more light carbon 12 is locked up in living organisms and organic matter being buried and preserved in sedimentary rocks. Consequently, the atmosphere and oceans become depleted in carbon 12 and enriched in carbon 13.
The carbon isotopic ratio in calcareous shells of marine organisms is in equilibrium with that of seawater. So as more carbon 12 is held in biomass during times of high primary productivity, and increased burial of organic carbon, calcareous (CaCO3) skeletal materials become enriched in carbon 13. During periods of low biological productivity and decreased burial of organic carbon, for example following mass extinctions, marine calcareous skeletal materials become enriched in carbon 12.