Weathering
When rocks (igneous, sedimentary, or metamorphic) are at or near the surface of the earth they are exposed to the processes of weathering.
In mechanical weathering rocks are broken up into smaller pieces by frost-wedging (the freezing and thawing of water inside cracks in the rock), root-wedging (tree and other plant roots growing into cracks), and abrasion caused by, for example, sand-blasting of a cliff face by blowing sands in the dessert, or the scouring of water transported sand, gravel, and boulders on the bedrock of a mountain stream. Mechanical weathering breaks rocks into smaller and smaller pieces but without otherwise altering the minerals.
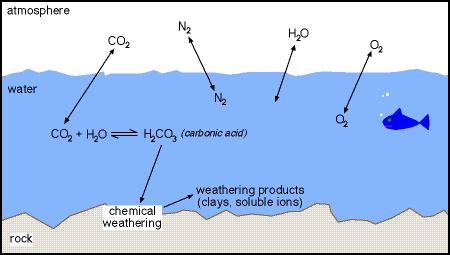
In chemical weathering minerals are changed into new minerals and mineral byproducts. Some minerals like halite and calcite may dissolve completely. Others, especially silicate minerals, are altered by a chemical process called hydrolysis. Hydrolysis is the reaction of minerals in weakly acidic waters. Most natural surface waters are slightly acidic because carbon dioxide from the air dissolves in the water. Some of the dissolved CO2 reacts with the water forming the chemical compound carbonic acid.

The common rock forming silicate minerals (except quartz) weather by hydrolysis to form:
Cations are positively charged ions; ions are charged atoms with too many or too few electrons. The cations include the iron, magnesium, aluminum, sodium, potassium, and calcium ions that form the common silicate minerals along with silicon and oxygen.
Soluble silica (H4SiO4) and metal cations (e.g., calcium, Ca++; sodium Na+; iron, Fe+++) are dissolved materials that become part of the water, like the dissolved minerals listed on a bottle of mineral water. Clay minerals are very small (sub-microscopic) solid particles. They are sheet silicate minerals like micas. Quartz chemically weathers only very, very slowly because of its high stability. It is mostly just broken down into small, sand-sized and smaller particles.
Complete weathering of silicate rocks will yield:
|
solid materials |
1) clays |
|
|
2) quartz sand (if the rock originally contained quartz) |
|
dissolved materials |
3) soluble silica |
|
|
4) metal cations |
Rock fragments will also remain where the rocks are not completely weathered.
Goldich (1938) studied the mineralogic changes of granitoid rocks during weathering. From this study he was able to establish the chemical weathering stability series shown below.
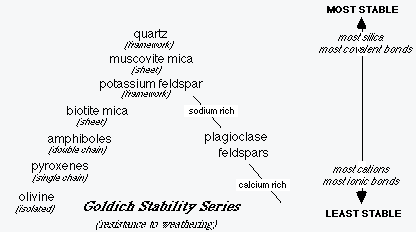
Chemical weathering reactions are with the cations that bind the silica structural units together. So it makes sense that isolated tetrahedra are the least stable in weathering, while quartz, which is completely formed of interlocking silica tetrahedra with no intervening cations, is the most stable.
Not only is quartz the most stable of the common rock forming minerals in chemical weathering, its high hardness and lack of cleavage make it quite resistant to mechanical weathering. Quartz is itself an agent of mechanical weathering in the form of blowing dessert sand.