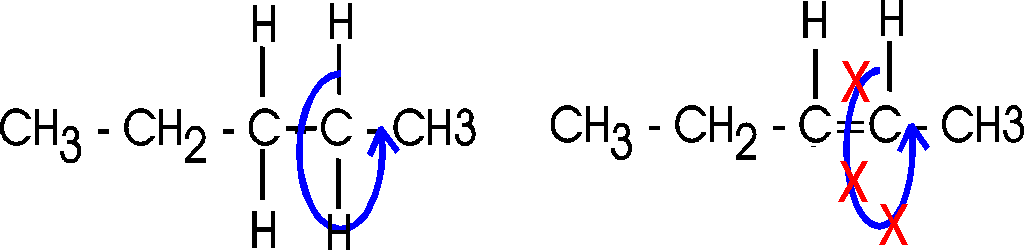Last updated:
September 14, 2010 02:43 AM
with updated fig refs to Purves 7th edition
© Copyright 2010 Lawrence Chasin and Deborah Mowshowitz
Department of Biological Sciences Columbia University
New York, NY
Bio C2005/F2401x 2009
Lec.3 L. Chasin September 14, 2010
Props: tinker toys. molecular
models for amino acids,
See rec. section assignments on website.
Recitations start this week. Quizzes start next week for C2005 students.
Anyone who needs to change or still sign up for
a recitation section, email Jessie at jrk2159@columbia.edu
--------------------------------------------------------------
POLYSACCHARIDES.
Glucose ring formation
Starch and cellulose: function follows form
LIPIDS: Soluble in organic solvents
Steroids
Fats: Fatty acids and glycerol as monomers
Esters; fats vs. oils; saturated vs. unsaturated fats
Phospholipids, phosphoesters, phosphatidylcholine
Phospholipid bilayer; membranes
Proteins: monomers = amino acids
pK
Stereoisomers: L and D amino acids
Polypeptides
Peptide bonds
Primary structure

(Ring formation)
In 3-dimensional space, a hexose chain can
easily curl up, such that the oxygen attached to carbon 5 can be juxtaposed next
to carbon 1. A 6-membered ring forms preferentially in water,
by attack of the hydroxyl of carbon-5 (C5) on the carbonyl double bond at C1.
One bond of the carbonyl double bond opens up and forms a new bond between
carbon-1 with the O of C5. The H leaves C5 and a new OH group is formed on
carbon 1. Follow along with the diagram on the
glucose handout, So a 6-membered ring is formed, with O as
one of its members (one of the vertices). One carbon (C6) is left sticking out
away from the ring. Unlike most biochemical reactions, which require a catalyst
to help them take place at a reasonable rate (more on this in a week or so),
this intramolecular cyclization reaction takes place all on its own, as soon as
a sugar is put into a water (aqueous) solution. This reaction is rapid because
the players can't help but keep bumping into each other as the glucose chain
dances in thermal motion. The ring structure can also open up, re-forming the
straight chain. The 2 forms are in a dynamic equilibrium, but because the ring
form is more stable, this species predominates in water.
{Q&A}
(Anomers: alpha and
beta glucose)
Now, when the O attached to C5 approaches
the carbon C1 which has the carbonyl double bond, it can do so from one side or
from the other side. Depending on which side is attacked, the resulting ring
comes out looking different in 3-dimensional-space, because the OH formed from
the carbonyl oxygen is oriented distinctively in the 2 cases. That is, the
resulting ring can be of
two different conformations in space. The two conformations are formed at
roughly equal frequencies. The 2 conformations are called alpha and beta:
Alpha, where the
C1 OH that is formed ends up BELOW* the C1 hydrogen,
or
Beta, where the C1 OH that is formed ends up ABOVE*
the C1 hydrogen (see
glucose handout, right side. See also a picture
[Purves 3.11]).
This relative position information can be seen
in the Haworth depictions in which the glucose ring is depicted as flat (right
sides of handout 2-7 and 2-8).
* "Above" and
"below" refer only to the Haworth projection depiction and not real
three-dimensional space, see blow.
{Q&A}
{Q&A}
(Chair form)
The ring is actually not flat, but puckered into a reclining chair-like shape,
but hard to draw: (see
flat vs. puckered handout 2-8) - - -in this chair-view the hydrogens and
the hydroxyls can be seen to be not really up or down, but are rather either
axial (vertical = sticking up OR down) or equatorial
(horizontal = sticking out).
Note in glucose all the hydroxyls are
equatorial except that of the #1 carbon in the alpha conformation. In
beta-glucose this -OH is upper, relative to the hydrogen, and is in fact
equatorial; but in alpha-glucose it is lower (relative to the hydrogen) and
axial (and down).
You should study handouts 2-7 and 2-8 until you
are satisfied that you can picture these chair conformations in 3-dimensional
space.
The existence of these two seemingly very
similar 3-dimensional structures for glucose can have important effects on the
3-dimensional structure of polysaccharides made from these glucose monomers,
which in turn can determine the function of the polysaccharide, as we will see.
Glycosidic bonds)
As we consider a polymer built from glucose
monomers, we can first consider a dimer. Two glucose monomers can be connected
to form a DIMER. This connection, WHICH DOES NOT HAPPEN BY ITSELF (i.e., without
some help from a catalyst), involves a dehydration, the removal
of one molecule of water, from the 2 monomers:
2 monomers ----------------------> dimer
R-OH + R-OH ---------> R-O-R + HOH
This type of reaction is also referred to as a
CONDENSATION, as it condenses two molecules into one.
The resulting -C-O-C- bond is called a
glycosidic bond when it is connecting two sugars.
Conversely, the breakdown of polymers back to
their constituent monomers involves the reversal of this chemistry, the addition
of water, or hydrolysis (the products = a hydrolysate).
R-O-R + HOH ------> R-OH + R-OH
{Q&A}
{Q&A}
Both of these reactions require different
catalysts in the cell in order to occur, which is generally true for all the
biochemical reactions we will discuss. See the
carbohydrates handout (2-6), below the line, for a depiction of two dimers in the
flat ring forms. Note the 1-4 linkage (C6 sticks out of the ring, so that
is one way to figure out the numbering in the ring). Although the bonds are
presented as bent at sharp angles, they are not really so, it is just a way of
presenting both sugar monomers right side up and still connect them with a
glycosidic bond.
These dehydrations can continue in many cases
in a repeated way to form chains that contains 1000's of monomers. E.g.,:
X--1,4--X--1,4--X--1,4--X--.........(where X
represents a sugar ring)
To be sure you understand
disaccharides, try Problem 1-8C and 1-9 D & E.
(Starch and cellulose:
function follows form)
If glucose molecules are put together with
an alpha 1,4 link, then the 2 sugars in the dimer lie at an angle relative to
each other. Here, the
C1 -OH is axial whereas the C4 -OH in glucose is (always is)
equatorial. The disaccharide in this case is called maltose. The angle of this alpha 1,4 bond is such that the polymer bends at
each glycosidic connecting bond, as can bee seen in
handout 2-9, disaccharides in chair form. As a result, it takes on a
helical shape that allows lots of hydrogen bonding between glucoses in
each turn of the helix, thus stabilizing the polymer in this shape.
[TINKER TOY demo]. Such is the case with STARCH,
which consists of alpha-glucose molecules joined in 1,4 linkages. In addition,
starch has branches
[Purves 6:3.14b, 7:3.16] made by linking additional glucose molecules at the C6 OH of
some of the glucose residues in the chain, via an alpha 1,6 bond). The branched
chain
continues with alpha 1,4 linkages (see Becker 7th: Fig. 3-24). The length and
frequency of the side chains give rise to the different forms of starch
(potatoes, corn) or of a starch-like polymer found in mammalian liver and
muscles,
GLYCOGEN (and see
[Purves 6:3.14a2, 7: 3.16] . These polymers act as storage forms for glucose. When
glucose is needed, they can be hydrolyzed (adding water back to the bond between
the monomers) to regenerate the free monomer. Glycogen is more highly
branched than starch, and its breakdown from the many ends so produced leads to
rapid mobilization of the glucose moieties within it, a property more important
in animals than plants.
{Q&A}.
When a catalyst (a different one) puts together
glucose molecule that are in the beta conformation, polymer of a different shape
results. Here the disaccharide is called cellobiose. A poly-glucose of this type is
CELLULOSE, which contains exclusively glucose
molecules in beta linkages The beta linkage is a pretty straight
connection between the C1 and C4 of adjoining carbon atoms, since they both are
equatorial and so are sticking out, as can be seen on
handout 2-9, disaccharides in chair form. Thus a cellulose chain extends
straight with its C6 OHs sticking out from the chain on either side.
[Purves 3.14a1] Many cellulose molecules can then associate side by side
(via hydrogen-bonds to each other) to form a fiber of great strength (e.g., in
cotton, and it also contributes to rigidity of wood )
[Purves 6:3.14b, 7:3.16] [TINKER TOY demo]. Although the hydrogen bonds that
hold the cellulose polymers together in a bundle are individually weak, because
there are hiundreds or thousands of them, the bundle stays together. At
any moment some will be broken but others will not, so there is strength in
numbers here. Cellulose is the most abundant carbon
compound in the biosphere, accounting for about half of all such carbon.
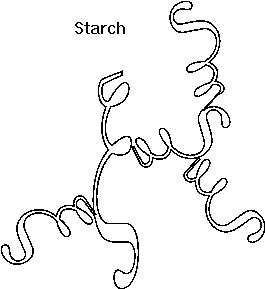
Here is our first good example of an important
theme in biochemistry, the relationship between structure and function at the
molecular level. The straight linear structure of cellulose made possible by the
beta-linkages allows the assembly of thousands of aligned molecules to produce a
cellulose fiber of great tensile strength. The alpha-linkage in starch produces
a compact structure, not strong, which serves as a storehouse of glucose for
energy when needed.
Your texts have additional examples of
important polysaccharides. Some of the sugars have nitrogen-containing groups
appended to the basic carbohydrate ring. The rigid bacterial cell wall is
another example, like cellulose, of a polysaccharide used for structural
support. So is the shell, or exoskeleton, of insects (CHITIN)
[Purves 6:3.15c,.7: 3.17].
To go over the structure of polysaccharides, try problem 1-11. If you need more review, try 1-25.
There are several different hexoses in most
cells. Fructose, galactose, and mannose are some common ones. Differences lie in
the positions of the carbonyl along the chain and relative positions of the
hydroxyls in space. Fructose has a ketone carbonyl at C2 which is its anomeric
carbon; it cyclizes to form a
5-membered ring (still with one member oxygen, of course, so 2 CH2OH
groups stick out
from the ring (Carbohydrates
handout).
And there are several common disaccharides (as
distinct from polysaccharides) found in nature (see
Becker 7th Fig. 3-23):
Glucose-glucose via a 1-4 alpha-link is
maltose, where alpha refers to the state of the -OH
in the monomer joined at its C1 carbon
[Purves 3.13a]. Maltose is formed as you digest bread.
{Q&A}
Galactose + glucose
[Purves 6:3.12, 7:3.14] via a 1-4 beta-link is lactose
(in milk) ,
Glucose + fructose
[Purves 6:3.12, 7: 3.14] via a 1-2 alpha-beta link = sucrose
(table sugar).
(LIPIDS:
Soluble in organic solvents)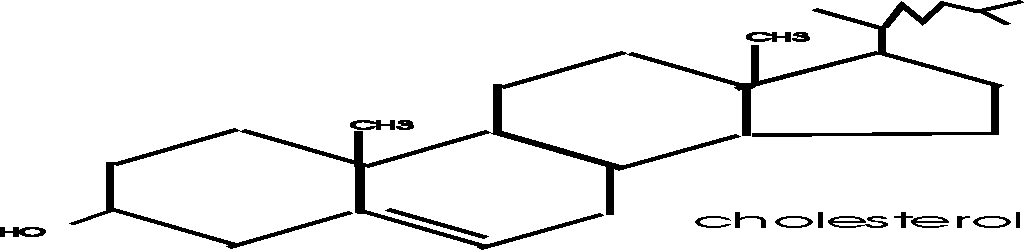
LIPIDS:
This is a more heterogeneous group, being defined as substances in a cell that
are extractable in organic solvents. Non-polar compounds are not soluble in
water, as they tend to coalesce. But they ARE soluble in non-polar solvents such
as octane or benzene (a hydrocarbon - compounds made up of
just hydrogen and carbon atoms, like the octane molecule we considered earlier).
So lipids are molecules that have extensive non-polar regions.
(Steroids)
Steroids such as cholesterol and testosterone have multiple hydrocarbon rings,
and are in this category
[Purves 6:3.24, 7: 3.23], (see Becker 7th: p.70). Note the drawing conventions, with further
shorthand: the depiction (not) of C's and H's. C is assumed to always have 4
bonds; unless otherwise specified, C's are assumed to be present at the vertices
on drawn bonds; bonds from such C's that are not shown are assumed to be to H,
to make a total of 4. Almost no atoms are named, yet the structure is
completely defined.
Steroids are small molecules that are
not monomers, they do not become connected to form polymers. Cholesterol is a
steroid that is a component of the cell membrane, which we discuss in a few minutes.
Steroids such as testosterone, estrogen, cortisone, and vitamin D are hormones,
compounds that circulate in the blood to send signals from cells in one part of
the body into cells in another region. You will learn more about steroids in the
physiology section in the second semester.
(Fats: Fatty acids and
glycerol as monomers)
A major class of lipids are the fatty
acids, long straight chain hydrocarbons with a carboxyl group (carboxylic acid)
on one end. See
[Purves 6:3.19, 7:3.18], and another
picture.
{Q&A}.

(Esters; fats vs.
oils; saturated vs. unsaturated fats)
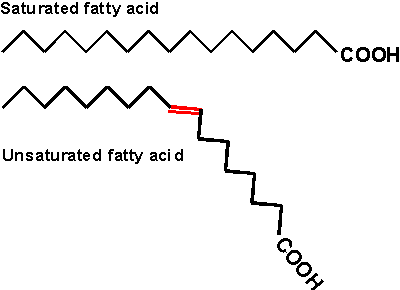
Inside cells, fatty acids (FA) are usually connected to a molecule of the
tri-hydroxy (tri-alcohol) compound glycerol. Once again water is removed, this
time producing an ester bond (acid
+ alcohol, see top right corner of
lipids handout 2-10). If all 3 OH 's on the glycerol are
substituted with FA's, then we have a triglyceride. See
[Purves 6:3.19, 7: 3.18], and another
picture. This is fat. Fats are very hydrophobic and
are practically insoluble in water. You can also have mono- or di-substituted
glycerol, but it is the triglyceride that is fat. Fats differ according to the exact nature of the FA's that are
present. "Saturated" fats have -CH2- (methylene) groups, usually
18-20 of them, along the chain. They are saturated with hydrogens,
compared to the unsaturated variety. The latter may have a double bond or two within
the chain, and thus have less H's (unsaturated for H's). The presence
of the double bond puts a crimp into the structure, since unlike single C-C bonds,
there is no rotation about C=C double bonds)
[Purves 6:3.20, 7:3.19]. As a result, it is more difficult for the unsaturated fatty acid molecules to
associate.
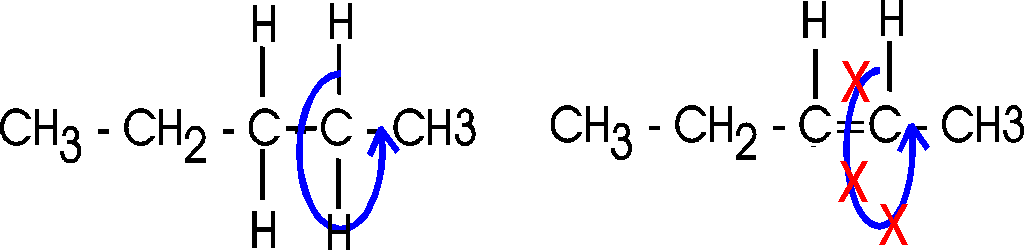
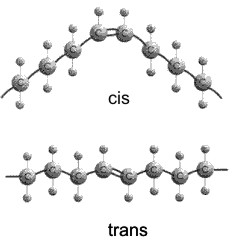
Actually, there are 2 ways a double bond can
form in a fatty acid, called cis and trans. In the cis case, the two
hydrogens are on the same side of the double bond (remember there is no
rotation, so their position is fixed). Now the two bonds carrying the rest of
the carbon atoms are also together on one side of the double bond, so the
molecule is crimped, with a severe angle between the two hydrocarbon stretches.
In the trans case, the two hydrocarbon stretches are on opposite sides of the
double bond, and the overall chain is straighter. Most fatty acids in animals are saturated; with their relatively
straight chains their hydrocarbon chains are free to associate with each other
with no constraints and they aggregate into solid fat. Plants contain a
lot of unsaturated fatty acids of the cis type. These unsaturated fats (WITH the double bonds) are
usually liquids (oils), as their
crooked fatty acid chains cannot approach each other so easily. Take vegetable oil (unsaturated), and add hydrogen across
the double bonds and you get Crisco, or the creamy texture in peanut butter
(read the label: hydrogenated).
Trans fatty acids do occur made by
bacteria in the stomachs of ruminants like cattle, so they end up in beef to
some extent. A much greater source of trans unsaturated fatty acids comes
from the chemical hydrogenation of oils, where they are formed somewhat
ironically as a by-product of the hydrogenation process. The trans
unsaturated fats resist turning rancid, so are favored by the food industry.
However, they are more equivalent to saturated fatty acids in their ability to
form solid fat, which encourages the formation of atherosclerotic plaques.
Thus margarine may be as bad for you as butter. Or worse, as trans fats show a
stronger correlation with heart disease than saturated fat, possibly through
more indirect effects (e.g., membrane structure).
So here again as in the case of
polysaccharides, the 3-dimensional structure of the molecule has a lot to do
with its physical properties.
Fats are a good example of hydrophobic forces
at work. Just think of a fatty chicken soup with those globules of fat floating
on top, out of solution.
Fats serve as a storage form of energy. That
is, like glycogen or starch, fats can be broken down and used for energy
metabolism, as we will see later. Fats are stored in cells called adipocytes.
(Phospholipids,
phosphoesters, phosphatidylcholine)
There is a special class of lipids that are
related to the fats, but with a significant difference. These are the
phospholipids, an
example of which is shown in the middle of the
LIPIDS handout. Two of the glycerol hydroxyls are connected to long chain
fatty acids, but the third is connected to quite a different group, a phosphate.
Phosphoric acid (H3PO4) is an acid, The -OH groups
attached to the phosphorous easily lose hydrogens at neutral pH.
Phosphoric acid has 3
acidic hydrogens. [If you are shaky on pH, ask to review it in recitation
section.]
Phosphoric acid is a strong acid, losing most
of its hydrogen ions at pH7. The ion that is formed is called phosphate,
and we will treat the 2 names equivalently, considering them both acids
(referring to their origin as the acid). Similarly we will use carboxylic
acid and the carboxylate ion (the negatively charged unprotonated form)
synonymously in most situations.
The phosphate group is connected to a glycerol
hydroxyl, again by a dehydration that forms an ester (acid + alcohol). Whereas
up until now we had a carboxylic acid ester linking the fatty acid to the glycerol, here we
have a phospho-ester. The acid partner is the one named to specify a type of
ester. In both cases the ester is formed by an alcohol linked to an acid.
After linkage, the phosphate group is still charged, as shown.
The rest of the phosphate may be free, as in a phosphatidic acid,
or it may be esterified to yet another alcohol via another of its acidic groups;
a common one is ethanolamine: HO-CH2-CH2-NH3+.
The resulting phospholipid would be called phosphatidyl-ethanolamine, and
it would be categorized as a phospho-di-ester (phosphodiester). Note that the
presence of positively charged basic groups such as amines tends to neutralize
the negative charge of the phosphate, but only adds to the hydrophilic character
of the head of the phospholipid, by adding charged groups.
(Phospholipid bilayer:
membranes)
Phosphatidyl-ethanolamine is a compound
that is highly hydrophobic throughout most of the molecule, but then has a
highly polar group at one end, with two complete, if opposite, charges. A
further derivative has 3 methyl (-CH3) groups bonded to the
nitrogen instead of H's. This moiety is choline (tri-methyl-ethanolamine; the
nitrogen retains its positive charge. When esterified to a diglyceride one gets
phosphatidyl choline, depicted in [Purves
6:3.21, 7:3.20]. The polar end can interact strongly with water (it is hydrophilic),
while the remainder of the molecule wants to come out of aqueous solution. This
is a confused molecule. What happens is that the hydrophobic parts all line up
with each other to minimize their interface with water (both side-to-side and
end-to-end), while the charged ends remain in contact with water. See
[Purves 6:3.22, 7:3.21], and
photo. It is in this way that biological membranes form, as
a phospholipid bilayer, the charged ends of the double layer
being on the outside in contact with water, with the cytoplasm on one side and
the exterior of the cell on the other side: See
picture. And look in your textbooks for great diagrams of phospholipid
bilayers.
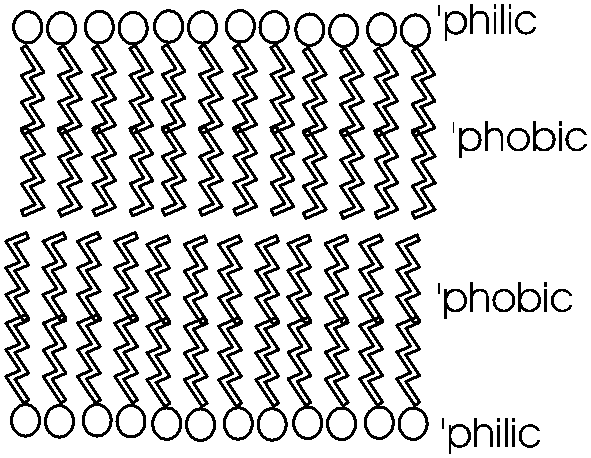
Such a bilayer presents a permeability barrier
to water-soluble compounds, which cannot pass through the hydrophobic barrier.
Special protein structures that are embedded in this membrane are then necessary to
allow the passage of water soluble compounds in and out of the cell. These are
the channels and pumps mentioned earlier. See a diagram of a cell membrane
at
[Purves 6:5.1, 7:5.1] and in your texts. Yet again we see how the chemical properties of these molecules
determine their structure, and how their structure provides a biological
function. To review phospholipid structure, try problems 1-12 & 1-13.
Large amounts of cholesterol are embedded in
the membranes of animal cells. The cholesterol is kept inside by
hydrophobic forces. It acts to plug spaces that could cause leakiness, to
impart more strength, and to prevent too much association of the saturated fatty
acids at low temperature (i.e., "freezing" of the membrane into fat).
The texts have nice diagrams of all this.
Lipids are impressive in their variety (see
picture) and especially in membrane formation, but admittedly they are not
really good examples of the linear biopolymers that we defined. But they have to
go somewhere, and so they are stuck amongst the macromolecules.
NUCLEIC ACIDS: Unlike the
catch-all category of LIPIDS, NUCLEIC ACIDS are
biopolymers par excellence. There are 2 types, DNA and RNA, the monomers are
nucleotides, that have nitrogen-containing rings, 5-carbon sugars, and
phosphodiester linkages. There are
four types of monomers in each polymer. We will discuss them in detail, but not
for a few weeks yet.
(Proteins:
Amino acids are the monomers (20))
PROTEINS. These are the most important class
of macromolecules in the cell, and we will discuss them now in detail. The
monomers that make up proteins are the amino acids, of which there are 20. The
same 20 in E. coli, in elephants and in eggplants.
The general structure of an amino acid is:
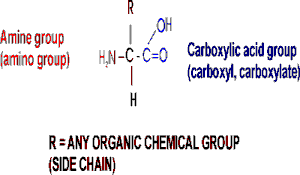
Note the central carbon atom, to which 4 different
groups are attached: an amino group (drawn by convention at the left), a
carboxylic acid group (put at the right side), a hydrogen, and a side chain, or
R-group. Only the R-group varies among the 20 different amino acids. This
is the side chain, and so there are 20 different side chains. Look at the
amino acids and peptides handout for some of the side chains. Your texts and
hard copy handout show all 20, and you should examine all 20.
Out of laziness, I drew the general amino acid
incorrectly: Actually at neutral pH, the molecule is charged, because the
carboxylic acid group is an acid, and the amine group is a base, so more
accurately: (also see
3-D picture)
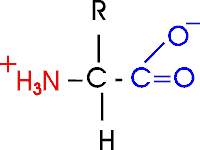
Let's take this opportunity to discuss the charge on
organic molecules a bit more. In living systems, the carboxylic acid group
is mostly charged and the amine is mostly charged, but that is at pH7, the
cellular pH under most circumstances. Is an acid always charged in aqueous
solution? No. It depends on the pH of the environment. In the laboratory we do
not have to keep things at pH 7, as it is in the cell. We can vary the environment at will, adding strong acids such as hydrochloric acid as a source
of hydrogen ions (lowering the pH), or a strong base such as sodium hydroxide
(raising the pH). The strength of an acid is a measure of how readily it gives
up a proton. Carboxylic acids are always in equilibrium with the hydrogen ions
(protons) in the solution, so if the hydrogen ion concentration is high (acidic)
then the equilibrium will shift toward the protonated (uncharged) species. At pH
2.5 an amino acid carboxyl group is protonated about half the time; for each pH
unit this proportion of protonated species will drop by a factor of 10, so very
little of the carboxyl group is protonated at the neutral pH of 7 found in most
cells. A similar situation pertains to the amine base end: at a very low H+ ion
concentration (e.g., 10-11 M H+, a high pH of 11), it will tend to
lose its extra proton, but at pH 7
it will mostly remain protonated, with a positive charge. There will always
be some intermediate pH at which we find the the group half charged/half
uncharged. This pH is called the pK of the group, and it can be influenced
by the remainder of the molecule. The pK is an indication of the acidic or basic
strength of the group (the lower the pK is the stronger the acid, the higher the
pK the stronger the base).
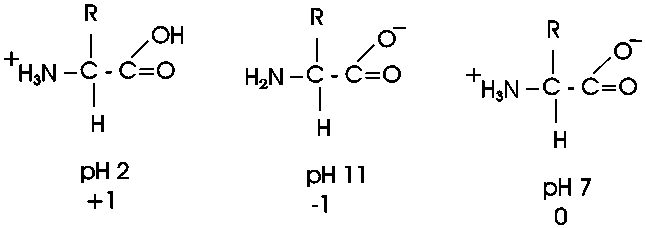
So at pH7, most amino acids are neutral (no net charge), but they are highly charged nonetheless.
{Q&A}
A molecule that is charged but electrically neutral is
called a zwitterion.
Now, what are some of these 20 different
side groups?
Here are 2 charged side group, e.g.:
asp: R= -CH2-COO- , there is a
second carboxyl group on this amino acid)
lys: R= -CH2-CH2-CH2-CH2-NH3+
, there's a second amine on lysine, so lysine will have 3 charged groups,
and a net charge of +1 (two +'s and one -) at pH7.
There is a convention for numbering amino
acid carbons; actually it's a lettering. It starts from the central carbon,
called alpha: so lys has (count with me) an alpha, beta, gamma, delta,
EPSILON-amino group as well as an alpha-amino group (and an alpha-carboxyl).
The average molecular weight of an amino acid is ~120,
but the range is from 75 to 203.
The smallest amino acid (a.a.) is glycine (gly), MW =
75. Here the side chain is merely hydrogen.
The largest is tryptophan (trp), MW = 203 [-CH2- bridge
to a 5-membered ring containing a N plus a fused 6-membered ring] and is fairly
hydrophobic.
Look over the structures of the 20 amino acids in the
textbook. It is the properties of the functional groups
on the 20 different side chains of the 20 different amino acids
that determine the function of a protein, so they are all-important. The
handout shows all 20 aa's, but without indicating the ionization of the acidic
and basic groups. We will discuss many of the side chains within the context of
the discussion as we go along.
There are two that deserve special mention: arginine contains a functional group that
will not be found elsewhere in this course; it is -NH-C+(NH2)NH2,
called the guanido group. The guanido group is a strong base, even
stronger than an ordinary amine, so it is positively charged at pH7 (like
lysine). You can consider the positive charge to be distributed over all 3
N atoms. Proline has a side chain that folds back and forms a covalent bond
to the amine nitrogen of the amino acid, thus producing a ring structure.
You should be able to recognize the properties of the
side chains as polar or non-polar, charged or not charged. You will not be
responsible for recalling a specific amino acid structure from the English name
or vice versa, but given the structure you should know how it behaves.
{Q&A}.
To be sure you understand amino acid structure, try problem 1-15 except C. For additional review of the effects of pH, try problem 1-16. For the effects of different R groups, try 1-20.
(Stereoisomers)
Now let's consider the structure of an amino acid in
3 dimensions:
When carbon forms 4 single bonds, it makes them spaced
equally apart from each other in space, in the form of a tetrahedron as in this
representation of glycine [a model with 2 white groups (the H's) is shown].
Now consider this other molecule of an amino acid [again
with 2 white groups], with 2 H's of glycine, e.g. Are these the same molecule,
that is, are they distinguishable or are they indistinguishable?
They are indistinguishable, since I can rotate them and
superimpose their atoms.
But now suppose I make this alanine instead of glycine.
I replace one white H with a -CH3 group [orange] on each molecule [I am being sure to
make them stereoisomers].
I can no longer superimpose them. They are both alanine,
as they have the same four groups attached to the central carbon. But in three
dimensions they are actually mirror images of each other. See
[Purves6ed+7th: 2.21a]. We call one D-ala
and one L-ala. See [Purves6ed+7th: 2.21b].
This one is D, or is it this one... ? I can't remember
.. it's not too important here.
What is important is that in general, you have this
situation, the possibility of two stereoisomers,
whenever there is what is called an ASYMMETRIC
carbon atom in a molecule, that is, a carbon with four different groups
attached. No matter how you might divide an asymmetric molecule in two by
placing a plane through it, the two sides look different.
These stereoisomers are sometimes called optical
isomers, since the two forms, in solution, will bend a beam of polarized light
one way or the other. Thus the D designation originally meant dextro, for to the
right, whereas L stood for levo, to the left.
All amino acids except glycine have an asymmetric
carbon, which is the alpha-carbon. So we can draw 19 of the amino acids in 2
stereoisomeric forms.
So do we really have 39 a.a.'s? No. All the
stereoisomeric forms of the amino acids in proteins are
L-amino acids, so we only have to worry
about 20.
Note that the sugars we discussed, like glucose,
have several asymmetric carbon atoms. Aside from L and D designations, the sugar
stereoisomers are given different English names (e.g., D-glucose, D-mannose,
L-rhamnose, etc.).
To review the various chemical groups discussed so far, try problem 1-19.
(Polypeptides, peptide bond)
Polymerization of aa's
OK, now let's now string these L-amino acids together, polymerize them. The
bond that connects two amino acids is an AMIDE bond
(-CO-NH-) between the carboxyl of one amino acid and the amino group of the
next. Once again, a molecule of water is removed in the formation of the
connecting bond:
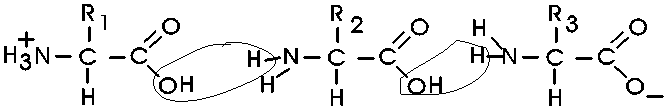
In the special case of proteins, this amide bond is called
a PEPTIDE BOND, and the resulting product a PEPTIDE,
a dipeptide (or we could go on to a tri-peptide, oligo-peptide, or finally,
POLY-PEPTIDE). (See also
polypeptide handout). Also see another
picture. {Q&A}
By convention, the amino group is written on the left
for an amino acid and also for a peptide.
In the tripeptide in the diagram, note the peptide bond
(boxed), and the repeating unit, or aa "residue" [circled]. Residue refers to
what's left of the amino acid monomer after it has been incorporated into
a polypeptide, which is most of it: it just lacks one H at what was the amino
end and one OH at what used to be the carboxyl end. Note also that the charged
amine and carboxyl groups no longer exist inside the polypeptide, having been
replaced by the amide, an uncharged (but polar) functional group.
Almost all polypeptides have 2 ends, the
amino end and the carboxyl
end, which do remain charged at pH7.
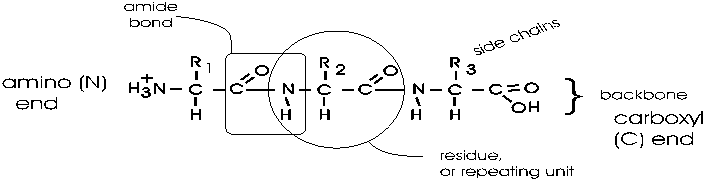
The "backbone" of the
polypeptide is defined as all of the atoms except the side chains.
The only free amino and
carboxyl atoms of the backbone are at the 2 ends.
The side chains , then, stick out of this backbone (also
see
polypeptide handout).
Nomenclature: e.g., alanine-methionine-alanine, or
ala-met-ala, or alanyl-methionyl-alanine, or AMA
To review peptide structure, try problem 1-15, C, and then try 2-3 part A.
The length of polypeptides is commonly 100-1000 amino
acids, but smaller and larger ones also can be found.
Each and every protein molecule in the cell has an
identity defined by its particular sequence of amino acids. Each E. coli cell
contains about 3 million polypeptides molecules, but only about 3000 different
ones. Each of these individual protein types has a name to go along with its
chemical identity.
Are there enough combination to specify 3000 different
polypeptide sequences? Well, if the average polypeptide is 500 amino acids
long, then the possible combinations are 20500 or 10650,
which is a number of inconceivable magnitude. So evolution has settled on about
100,000 of these combinations to do biological jobs.
Some examples of polypeptides, taken not from E. coli,
but from more familiar organisms include:
Carriers: hemoglobin, which carries oxygen in red blood cells;
Nutrients: egg albumin, a nutrient in the white of a hen's egg;
Structural: keratin, providing toughness in skin, fingernails, and
wool;
collagen, providing a strong connection between cells in
tendons;
Signal reception: estrogen receptor (intracellular)
epidermal growth factor receptor (spanning the cell membrane)
Recognition of foreign substances: immunoglobulins
(antibodies)
Enzyme catalysts: beta-galactosidase, which helps digest the milk sugar
lactose.
We will discuss enzymes in some detail as an important
category of proteins.
(Primary (1o)
structure = linear
sequences of AAs)
Each of these proteins contains a polypeptide with a particular sequence of
amino acids, usually all 20 are represented, although not at all equally. Unlike
polysaccharides, this sequence usually exhibits no obvious simple regularity, or
repeating subsequence:
This linear sequence of amino
acids is called the primary (1o) structure
of a protein. {Q&A}
It might be, for instance: met-ala-leu-leu-arg-glu-leu-val ...... How
is this sequence determined?
© Copyright 2010 Lawrence Chasin and Deborah
Mowshowitz Department of Biological Sciences Columbia
University New York, NY