Ultrafast Excited State Electron Transfer at the Orangic Liquid/Aqueous Interface
Related Papers
|
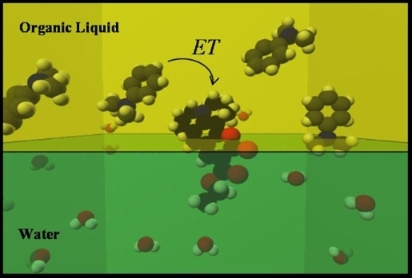
Immiscible liquid interfaces are important throughout many areas in chemistry and biology. The asymmetric nature of this environment lends itself to the unique chemical behavior which is found there. Hence, there is considerable fundamental interest in obtaining detailed molecular information on photophysical and photochemical events in these environments.
Taking advantage of the inherent surface sensitivity of Second Harmonic
Generation (SHG) we have been able to obtain time resolved excited state
electron transfer (ET) dynamics at the Dimethylaniline (DMA)/Water interface.
|
|
By directly monitoring the formation and decay of the radical cation
produced by this process, we find that ET occurs on a 10-12 second time
scale. Our continuing study of ET at the interface is providing us with a detailed
molecular picture of this event in this environment. The "real time" picture developed
from this study will help improve theoretical models and directly
impacts areas such as interfacial synthesis, chemical sensors, drug
delivery pharmacology and the impact of hazardous environmental
contamination.
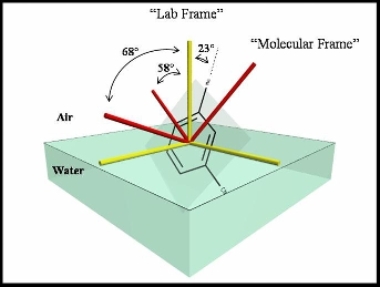
Absolute Orientation of Molecules at Interfaces
Related Papers
|
Molecular orientation at surfaces and interfaces is of widespread interest in science. This topic has relevance to chemical reactions, long and short-range molecular interactions, phase transitions and the electronic and vibrational states of interfacial molecules. Sum Frequency Generation (SFG) is inherently sensitive to molecular vibrations if one of the incident beams is infrared. This configuration allows the measurement of orientation of two different SFG active chromophores with respect to the surface normal. These two measurements allows us to obtain absolute orientation defined by both symmetry axes.
|
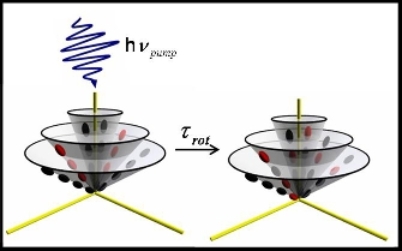
Molecular Rotations at Interfaces
Related Papers
Molecular interactions and motions at the interface are different from those of the bulk liquid due to both the change in solvent properties at the interface and the inherent anisotropy of the interfacial environment. The time varying torques exerted by solvent molecules are anticipated to be different at an interface compared with the bulk liquid. The origin of these differences is the change in density and the orientational dependence of the intermolecular potential at the interface. These factors effect an anisotropic orientation and a preferred alignment of adsorbed molecules and a solvent structure unlike that of the bulk liquid.
|
|
Through our current studies of coumarin molecules reorienting at the Air/Water interface we have found that rotational times are slower indicating friction is higher at the interface. The reason for this is that water molecules at the interface form more ordered hydrogen bonding networks than in the bulk water. We have further been able to separate in-plane and out-of-plane motions. We find that the in-plane motion is 20% faster than out-of-plane. Currently we are exploring the effects of electrostatic charge and isotope effects.
Absorption, Surface Potential and Charge Density of Colloidal Interfaces
Related Papers
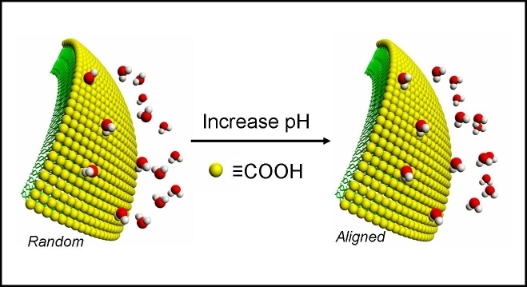 |
Interest in colloidal systems are ever increasing both from the viewpoint of fundamental science, and due to their growing applicability in biomedicine. Colloids, as they are often called, are significant in the realm of basic sciences because they are closely related to surface chemistry. This is attributed to the fact that colloidal particles exhibit a large surface are to volume ratio. Hence, chemistry associated with colloids, dispersed in a fluid medium, is essentially influenced by the surface properties of the particles.
|
In addition to this fundamental importance of colloidal system in relation to surface chemistry, application of colloids in the field of biomedical science has also been proven to be of great value. For instance, bio-molecules can be tethered to the surface of various functionalized polymer colloids. These modified particles then serve as carriers of specific bio-molecules such as antigens and antibodies, as stimulants of the immune responce in immunoassays, and if fluorescently labled, as very sensitive detectors for protein molecules. Consequently, colloids have gained special interest in the issues concerning medical diagnostics and rug delievery systems. Application of colloidal systems is also prominent in the areas of environmental and material science as well as in chemical manufacturing.
Using the technique of SHG we studied adsorption of molecules at the latex particle surface, acid-base chemistry at the carboxylated polystyrene particle surface, surface potential and charge density meaurements of various colloidal systems. With advances in material science there now exists many different types of colloidal systems with diverse physical and chemical properties, which we wish to investigate using the surface selective method of SHG.
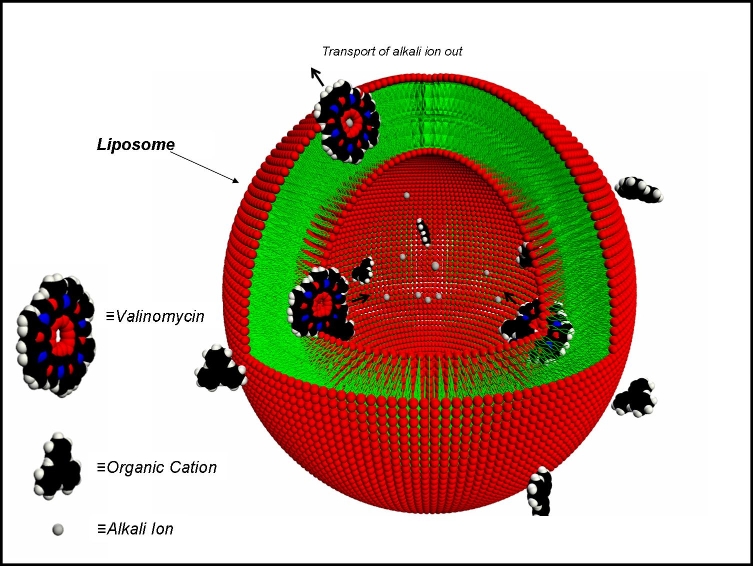
Antibotic Assisted Molecular Ion Transport Across a Membrane in Real Time
Related Papers
| An issue of special importance in biology and medicine is the time it
takes chemical species to cross the membrane of a cell. The transport
of an organic cation across the membrane mimetic bilayer of a liposome
has been observed in real time. The spectroscopic method that made it
possible to observe the kinetics is SHG, which involves the conversion
of light at a frequency ω to light at a frequency 2ω. In the figure we
see that the crossing time is about 90s. By adding the antibotic
valinomycin which is known to carry alkali ions across biological
membranes, the electrostatic barrier, which limits the number of
molecules and the speed of crossing, can be eliminated. It is shown
that at a valinomycin concentration of only 10-8M, the speed
of crossing of the organic cation increases by a factor of three while
increasing the number that can cross to its maximum value. Many
applications of this new method can be anticipated.
|