:: Home > Research
Decision-making and voluntary movement selection
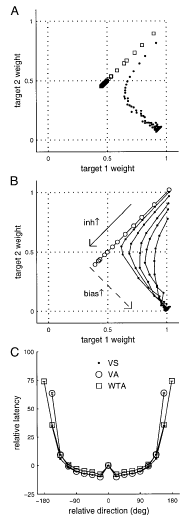 One of the main lines of research in my lab concerns decision-making in the context of sensorimotor behavior. Our view is that decision-making can be broken down into at least four subprocesses: signal detection, categorization, response selection and outcome evaluation. Much previous work in my lab focused on response selection in the oculomotor system. I started this work using the smooth pursuit system, which is an ideal model for investigating the interactions between motion processing, attention and voluntary movement selection. Steve Lisberger and I developed a behavioral paradigm for measuring eye movement responses to two or more moving targets. Using this paradigm, I have been able to characterize the process of target selection as a transition from vector-averaging to winner-take-all motor output (Ferrera 2000). I also found evidence for neural competition and cooperation in smooth pursuit target selection (Ferrera and Lisberger 1995, 1997; Ferrera 2000). Such models make interesting predictions about the role of recurrent inhibition in response normalization (averaging pursuit) and attentive selection (winner-take-all pursuit). One feature of the model is that, if there is no “bottom-up” bias in the sensory stimulus, the competition comes to a stalemate. To break the stalemate, there must be a “top-down” selection bias that favors selection of the target. It is possible that such a bias originates in prefrontal cortex (Ferrera et al. 1999; Opris et al. 2005).
One of the main lines of research in my lab concerns decision-making in the context of sensorimotor behavior. Our view is that decision-making can be broken down into at least four subprocesses: signal detection, categorization, response selection and outcome evaluation. Much previous work in my lab focused on response selection in the oculomotor system. I started this work using the smooth pursuit system, which is an ideal model for investigating the interactions between motion processing, attention and voluntary movement selection. Steve Lisberger and I developed a behavioral paradigm for measuring eye movement responses to two or more moving targets. Using this paradigm, I have been able to characterize the process of target selection as a transition from vector-averaging to winner-take-all motor output (Ferrera 2000). I also found evidence for neural competition and cooperation in smooth pursuit target selection (Ferrera and Lisberger 1995, 1997; Ferrera 2000). Such models make interesting predictions about the role of recurrent inhibition in response normalization (averaging pursuit) and attentive selection (winner-take-all pursuit). One feature of the model is that, if there is no “bottom-up” bias in the sensory stimulus, the competition comes to a stalemate. To break the stalemate, there must be a “top-down” selection bias that favors selection of the target. It is possible that such a bias originates in prefrontal cortex (Ferrera et al. 1999; Opris et al. 2005).
More recently, we have begun to look at the process of stimulus categorization using fMRI. Our approach focuses on categorization uncertainty as the main decision variable. We have subjects make simple perceptual judgments, for example, judging a line segment as long or short or judging a moving dot pattern as fast or slow. We use the subject’s psychophysical performance to quantify decision uncertainty. We can then look for BOLD activation that is correlated with decision uncertainty, while holding related variables, such as reaction time, constant (Grinband et al. 2006). One of the more exciting aspects of this research is that we have developed a system for functional imaging of awake behaving monkeys. We have trained monkeys and human to perform identical tasks (same stimuli, motor responses and rewards) and we are in the process of obtaining imaging data in both species.
Predictive mechanisms in prefrontal cortex
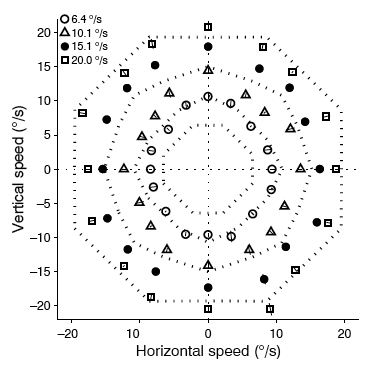 A very basic kind of short-range prediction is the ability to estimate the future location of a moving target that is temporarily hidden from view. Predicting the trajectory of moving objects is an essential navigational skill. The motivation for studying this type of prediction is several-fold. Voluntary eye movements - saccades and smooth pursuit - both make use of target motion information in a predictive manner. Voluntary eye movements are controlled, in part, by regions of the prefrontal cortex that receive robust innervation from motion-processing areas in parietal cortex. For the past few years, we have been recording from a part of prefrontal cortex called the Frontal Eye Field (FEF) to characterize signals related to the direction and speed of invisible moving targets. So far, we have found that about 1/3 of FEF neurons have directionally tuned responses that are sustained after the stimulus disappears. A similar proportion has speed-selective responses (Barborica and Ferrera 2003). These studies demonstrate the presence in FEF of signals that might support predictive eye movements by allowing reconstruction of the invisible target trajectory.
A very basic kind of short-range prediction is the ability to estimate the future location of a moving target that is temporarily hidden from view. Predicting the trajectory of moving objects is an essential navigational skill. The motivation for studying this type of prediction is several-fold. Voluntary eye movements - saccades and smooth pursuit - both make use of target motion information in a predictive manner. Voluntary eye movements are controlled, in part, by regions of the prefrontal cortex that receive robust innervation from motion-processing areas in parietal cortex. For the past few years, we have been recording from a part of prefrontal cortex called the Frontal Eye Field (FEF) to characterize signals related to the direction and speed of invisible moving targets. So far, we have found that about 1/3 of FEF neurons have directionally tuned responses that are sustained after the stimulus disappears. A similar proportion has speed-selective responses (Barborica and Ferrera 2003). These studies demonstrate the presence in FEF of signals that might support predictive eye movements by allowing reconstruction of the invisible target trajectory.
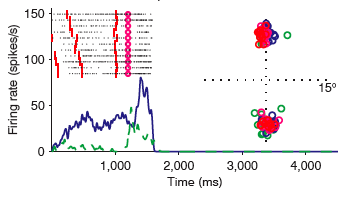 We are also investigating the predictive mechanism by electrically microstimulating the FEF. FEF stimulation produces involuntary saccades at low current thresholds. The direction and amplitude of the evoked saccade are characteristic of the stimulation site and there is a map of such “fixed vector” saccades similar to the map in the superior colliculus but a bit less orderly. Kustov and Robinson showed in the superior colliculus that the fixed vectors are not completely fixed but can be biased toward the locus of the animal’s spatial attention. This biasing effect also works for FEF and provides a method for tracking the animal’s attention while he is predicting the future location of the invisible moving target. We now have evidence that the animal’s locus of attention might actually move along the extrapolated path of the invisible target (Barborica and Ferrera 2004). This suggests that predicted movements to invisible targets are based on an internal representation that is dynamically updated, rather than being the result of associative stimulus-response learning.
We are also investigating the predictive mechanism by electrically microstimulating the FEF. FEF stimulation produces involuntary saccades at low current thresholds. The direction and amplitude of the evoked saccade are characteristic of the stimulation site and there is a map of such “fixed vector” saccades similar to the map in the superior colliculus but a bit less orderly. Kustov and Robinson showed in the superior colliculus that the fixed vectors are not completely fixed but can be biased toward the locus of the animal’s spatial attention. This biasing effect also works for FEF and provides a method for tracking the animal’s attention while he is predicting the future location of the invisible moving target. We now have evidence that the animal’s locus of attention might actually move along the extrapolated path of the invisible target (Barborica and Ferrera 2004). This suggests that predicted movements to invisible targets are based on an internal representation that is dynamically updated, rather than being the result of associative stimulus-response learning.
Spatial memory and updating in frontal eye field
Each time the eyes move, the map of visual space that is used for planning future movements must be updated. We investigated this process by training monkeys to perform a delayed spatial match-to-sample task (Balan and Ferrera 2003). During the delay, the animals were required to shift their gaze to one of four eccentric locations. Many frontal eye field neurons show significant delay activity selective for cue 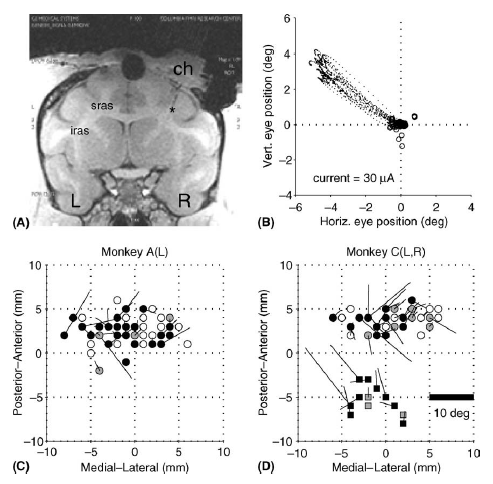 location. This activity is modulated when the animal moves its eyes. Despite this modulation, the neurons continue to signal their preferred cue location during most of the delay. However, after recentering saccades, the memory signal is temporarily abolished and then reemerges over a period of few hundred milliseconds. This is consistent with the idea that spatial working memory is buffered outside of the FEF. The modulation of neuronal firing seen when the monkey shifted its gaze is consistent with a vector subtraction mechanism that allows for the superposition of multiple saccade plans.
location. This activity is modulated when the animal moves its eyes. Despite this modulation, the neurons continue to signal their preferred cue location during most of the delay. However, after recentering saccades, the memory signal is temporarily abolished and then reemerges over a period of few hundred milliseconds. This is consistent with the idea that spatial working memory is buffered outside of the FEF. The modulation of neuronal firing seen when the monkey shifted its gaze is consistent with a vector subtraction mechanism that allows for the superposition of multiple saccade plans.
Several previous studies from other labs have suggested that a process of vector subtraction may play a role in spatial updating of movement plans. To further investigate the linkage between FEF activity and spatial updating, we electrically stimulated during the delay period of a memory-saccade task (Opris et al. 2005). The stimulation currents were subthreshold for evoking saccades. The electrical stimulus introduced a consistent bias away from the receptive/movement field of the stimulation site. In other words, the monkey behaved as if the electrical stimulus had evoked a small saccade toward the movement field. The effects of sub-threshold stimulation were consistent with a combination of vector subtraction and averaging, but not with vector summation. This experiment provided evidence that FEF may play a role in updating plans for memory-guided saccades when eye position changes during the memory period.
Research in the Ferrera Lab is funded by:
Alfred P. Sloan Foundation Fellowship (1997-1999) • Whitehall Foundation Research Grant (1997-2005) • Esther A. and Joseph Klingenstein Fund Fellowship (1998-2002) • McDonnell-Pew Program in Cognitive Neuroscience Research Grant (1999-2003) • EJLB Foundation Scholars Programme (1999-2002) • NIH-NIMH 1-R01-MH59244 (1998-2009) • NARSAD (2006-2008) • NIH-NIMH 1-R21-MH73821 (2007-2009)
