Abstract
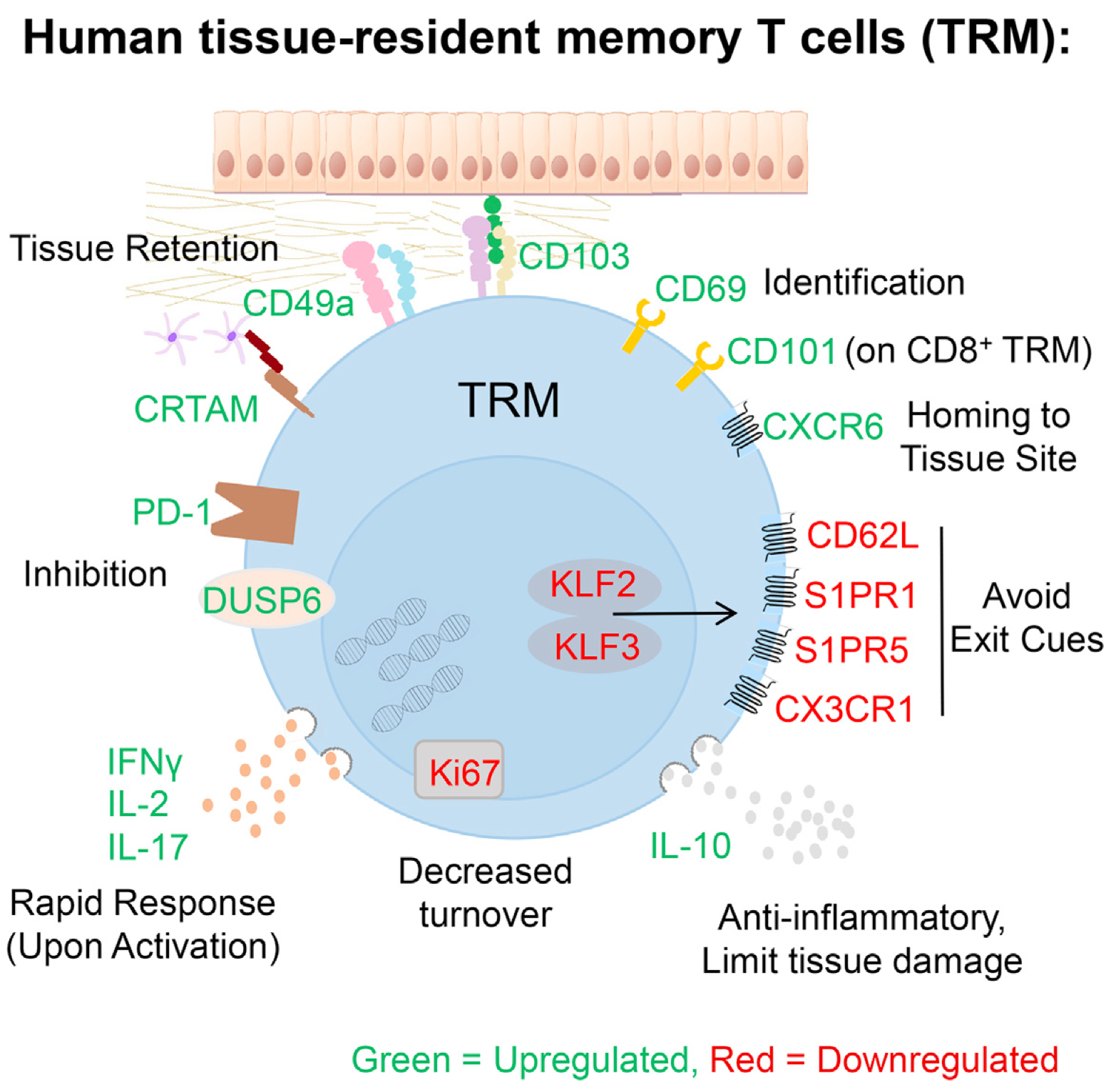
Tissue-resident memory T cells (TRMs) in mice mediate optimal protective immunity to infection and vaccination, while in humans, the existence and properties of TRMs remain unclear. Here, we use a unique human tissue resource to determine whether human tissue memory T cells constitute a distinct subset in diverse mucosal and lymphoid tissues. We identify a core transcriptional profile within the CD69(+) subset of memory CD4(+) and CD8(+) T cells in lung and spleen that is distinct from that of CD69(-) TEM cells in tissues and circulation and defines human TRMs based on homology to the transcriptional profile of mouse CD8(+) TRMs. Human TRMs in diverse sites exhibit increased expression of adhesion and inhibitory molecules, produce both pro-inflammatory and regulatory cytokines, and have reduced turnover compared with circulating TEM, suggesting unique adaptations for in situ immunity. Together, our results provide a unifying signature for human TRM and a blueprint for designing tissue-targeted immunotherapies.