Abstract
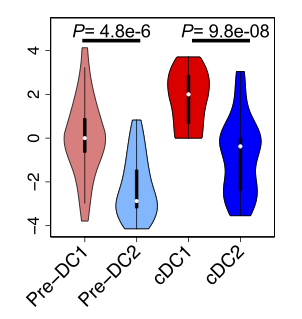
BACKGROUND: Classic dendritic cells (cDCs) play a central role in the immune system by processing and presenting antigens to activate T cells, and consist of two major subsets: CD141(+) cDC (cDC1) and CD1c(+) cDC (cDC2). A population of migratory precursor cells, the pre-cDCs, is the immediate precursors to both cDC subsets. Previous studies showed that there were two pre-committed pre-cDC subpopulations. However, the key molecular drivers of pre-commitment in human pre-cDCs were not investigated. RESULTS: To identify the key molecular drivers for pre-commitment in human pre-cDCs, we performed single cell RNA sequencing (RNA-Seq) of two cDC subsets and pre-cDCs, and bulk RNA-Seq of pre-cDCs and cDCs from human peripheral blood. We found that pre-DC subpopulations cannot be separated by either variable genes within pre-cDCs or differentially expressed genes between cDC1 and cDC2. In contrast, they were separated by 16 transcription factors that are themselves differentially expressed or have regulated targets enriched in the differentially expressed genes between bulk cDC1 and cDC2, with one subpopulation close to cDC1 and the other close to cDC2. More importantly, these two pre-cDC sub-populations are correlated with ratio of IRF8 to IRF4 expression level more than their individual expression level. We also verified these findings using three recently published datasets. CONCLUSIONS: In this study, we demonstrate that single cell transcriptome profiling can reveal pre-cDCs differentiation map, and our results suggest the concept that combinatorial dose of transcription factors determines cell differentiation fate.