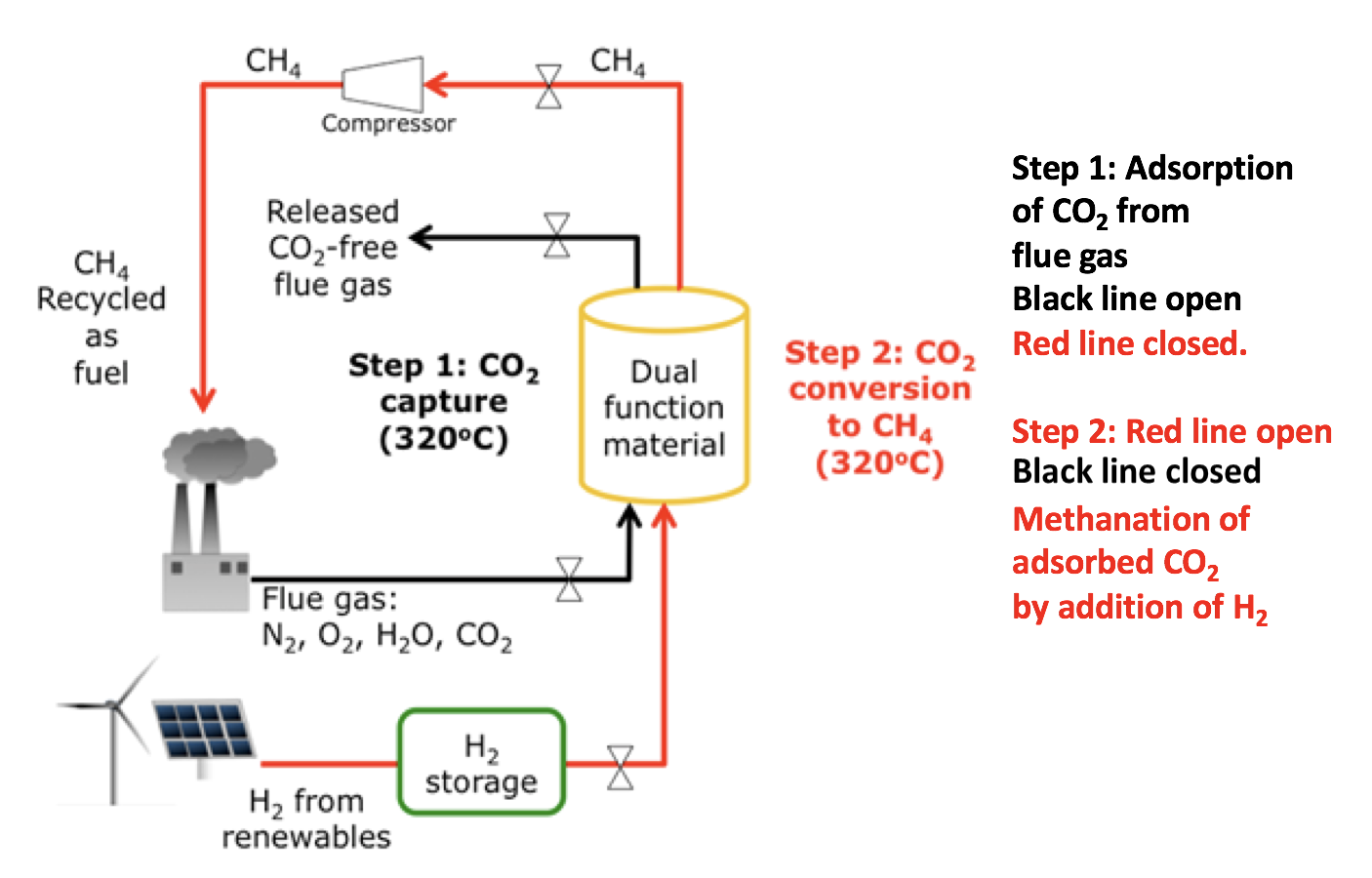
A Dual Function Material for the adsorption and conversion of CO2 to fuel
Anglo American Sponsorship, 09/2015 - PresentConventional CO2 capture and sequestration (CCS) is a very energy-consuming process in amine solutions (CCS) is very energy intensive. We have developed an advanced technology which adsorbs CO2 from flue gas and catalytically converts it to synthetic natural gas with our patented dual functional material (DFM). The DFM is composed of nano- dispersed CaO and Ru sites, respectively functioning as the CO2 adsorbent and methanation catalyst. For industrial applications, the influences of effluent gas environment (temperature, flow rate, O2, steam, composition, impurities, pressure drop and life) on the catalytic performance needs to be understood. The present project is focused on the scale-up study of CO2 utilization in various industrial applications. Various catalyst synthesis methods for industrial mass production, and reaction process parameters for optimum catalytic performance a are under investigation. Enhanced materials are also being sought.
- Martha A. Arellano-Treviño, Zhuoyan He, Malia C. Libby, Robert J. Farrauto, “Catalysts and adsorbents for CO2 capture and conversion with dual function materials: Limitations of Ni-containing DFMs for flue gas applications” Journal of CO2> Utilization Volume 31, May 2019, Pages 143-151
- Laura Proaño, EdissonTello, , Shuoxun Wang, Robert J. Farrauto, Martha Coboa, “In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru,“Na2O”/Al2O3 Dual Functional Materia,l” Applied Surface Science, Volume 479, 15 June 2019, Pages 25-30
- Shuoxun Wang, Robert J. Farrauto, Sam Karp, Ji Ho Jeon, Erik T. Schrunk, “Parametric, cyclic aging and characterization studies for CO2 capture from flue gas and catalytic conversion to synthetic natural gas using a dual functional material (DFM)” Journal of CO2 Utilization Volume 27, October 2018, Pages 390-397
- Other papers are under review
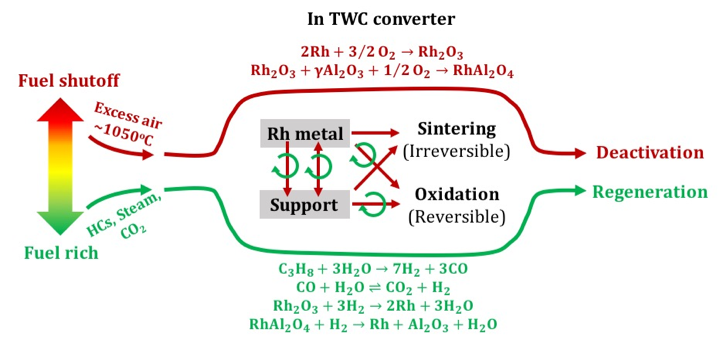
Regeneration of Rh and Pd TWC catalysts after automotive fuel shutoff
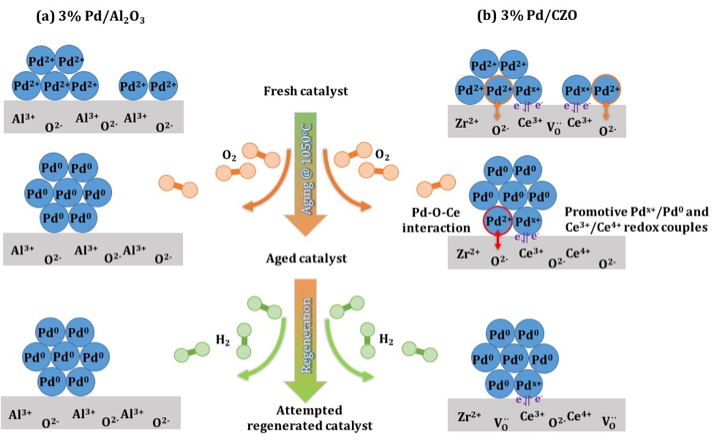
BASF Sponsorship, Columbia University, 02/2014 - 08/2015Modern three way catalysts (TWC) for automotive emission control, are composed of bimetallic Rh-Pd catalysts deposited on stabilized Al2O3. TWC experiences catalyst deactivation during fuel shutoff, an engine operational mode (e.g. coasting down hill) for enhancing fuel economy. A subsequent switch to fuel rich mode allows catalyst regeneration and the maintenance of catalyst performance. Different deactivation and regeneration effects were observed with supported Rh and Pd model TWCs. The research focuses on the influences of the two simulated processes on the Rh- and Pd-based TWC catalyst systems. The studies highlight the examination of catalyst redox chemistry, surface physicochemistry, and regenerability with metal-support interactions.
- Zheng, Q.; Farrauto, R.*; Deeba, M.; Valsamakis, I. Part I: A comparative thermal aging study on the regenerability of Rh/Al2O3 and Rh/CexOy-ZrO2 as model catalyst for automotive three way catalysts. Catalysts. 2015, 5(4), 1770-1796.
- Zheng, Q.; Farrauto, R.*; Deeba M. Part II: Oxidative thermal aging on Pd/Al2O3 and Pd/CexOy-ZrO2 in automotive three way catalysts: The effects of fuel shutoff and attempted fuel rich regeneration. Catalysts. 2015, 5(4), 1797-1814.
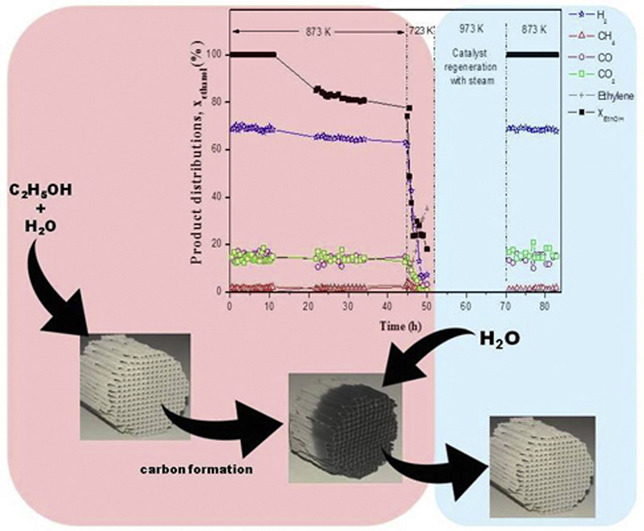
Reforming of ethanol to H2 for fuel cell applications: Rh/CeO2-SiO2 //ceramic Monolith
The performance of a new Rh/CeSiO2 catalyst supported on a ceramic monolith for steam reforming (SR) of ethanol for hydrogen generation was investigated. It provides several advantages over a traditional pellet based catalyst in that it will reduce weight, size and pressure drop in the reactor. The effect of steam to ethanol molar ratio and temperature were first investigated on a powdered catalyst in order to establish the preferred reaction conditions to be used for tests on the monolith. The optimum temperature for coke free, high selectivity and stable catalyst operation was 1073 K at a steam to ethanol molar ratio of 3.5. The monolith supported catalyst was evaluated for aging stability, on/off performance and coke regeneration using steam gasification. After 96 h of SR of ethanol at 1028 K and water/ethanol molar ratio of 3.5 the monolith supported catalyst retained stable performance throughout the entire time on stream with the only products being H2, CO, CO2. Some coke formation was observed using Raman spectra, however, it did not cause any permanent deactivation. Regeneration via steam gasification at 973 K with 20% steam in N2 was successful for coke removal and complete catalyst regeneration.
- Tamara S. Moraes, Luiz E.P. Borges, Robert Farrauto, Fabio B. Noronha “Steam reforming of ethanol on Rh/CeO2-SiO2 washcoated on a monolith: Stable catalyst performance,” International Journal of H2, Accepted
Structure dependence of Nb2O5-x supported manganese oxide for catalytic oxidation of propane: Enhanced oxidation activity for MnOx on a low surface area Nb2O5-x
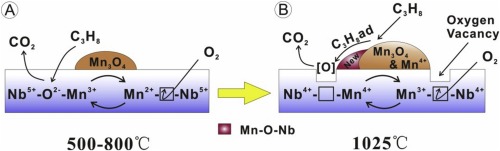
A series of Nb2O5-x with different structures were prepared as a carrier to manganese oxide catalysts for total oxidation of propane. The results demonstrated that a monoclinic structure of Nb2O5-x pre-calcined at 1025 °C, leads to significantly and surprisingly higher catalytic oxidation activity when MnOx is deposited at 400 °C even with extremely low specific area (around 3.94 m2/g) relative to the performance of MnOx/Nb2O5-x (∼>50 m2/g pre-calcined at 500 °C). Conversion vs temperature profiles for fresh and aged catalysts were generated and performance compared for different materials. Brunauer Emmett Teller (BET) and X-ray diffraction (XRD) were conducted to reveal the textural and structural features of niobium-based catalysts. Raman, X-ray photoelectron spectroscopy (XPS) and temperature programmed reduction (TPR) were performed to further understand the interaction between manganese and niobium oxides. Raman spectra indicated a new Nb-O-Mn species formed due to the strong interaction between the activated niobium oxide carrier at 1025 °C and manganese oxide. This study describes a synergistic catalytic oxidation effect between Mn oxides deposited on a specific phase structure of Nb2O5-x with very low specific surface area. The enhanced catalytic performance is directly related to the proper ratio of Mn3+/Mn4+ coupled with high ratio of Nb4+/Nb5+ on the surface and the oxygen vacancies generated between monoclinic Nb2O5-x and MnOx.
- YixuanWang, Sogand Aghamohammadia, Danyang Li, Kongzhai Li and Robert Farrauto, “Structure dependence of Nb2O5-x supported manganese oxide for catalytic oxidation of propane: Enhanced oxidation activity for MnOx on a low surface area Nb2O5-x”, Applied Catalysis B: Environmental Volume 244, 5 May 2019, Pages 438-447