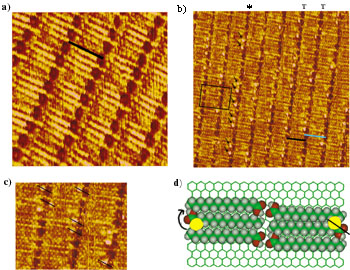
Left (a) An STM constant-current topograph of a hexadecanoic acid monolayer physisorbed onto a graphite surface taken at 1.5V (sample negative) and 300pA. This 8.5nm x 8.5nm image shows well-ordered lamellae separated by troughs, and a black bar represents a single molecule. These troughs consist of individual hydrogen-bonding carboxylic acid groups, which are identified as the dark circles. (b) through (d) show constant-current STM topographs of a 1:1 mixture by volume of 2-Br-hexadecanoic acid to hexadecanoic acid physisorbed onto a graphite surface, imaged at 1.5V (sample negative) and 300pA. (b)19nm x 19nm image displays hexadecanoic acid molecules (marked by a black bar) and an occasional (R)-2-Br-hexadecanoic acid molecule (marked by a blue bar), both of which are configured in the all-trans conformation. A trough is signified by a "T" and reveals dark regions that are attributed to the locations of hydrogen-bonding carboxyl groups accompanied sporadically by bright, topographical protrusions attributed to bromine atoms. All of the bromine atoms in the starred trough are signified by small arrows. The squared off region displays a small area where the interdigitation pattern, common to fatty acids, is apparent. The lamella axis is perpendicular to the molecular axis. An enlarged portion of (b) shown in (c) reveals a consistent orientation of bromine atoms relative to carboxyl groups; either the bromine lies above and to the left or below and to the right of the carboxylic group. Black lines superimposed onto some individual carboxyl/bromine combinations mark this orientation. The carboxyl - bromine - remainder of alkyl chain orientation is used to identify the 2-Br-hexadecanoic acid molecules in the domain as R chiral conformers of the molecule. A top view of a model of hexadecanoic acid interspersed by (R)-2-Br-hexadecanoic acid physisorbed onto the graphite surface is shown in (d). The black line superimposed on one of the bromine/carboxyl combinations mimics the arrangement found in the STM image (c). An arrow marking the clockwise direction of the bromine-carboxyl group - alkyl group orientation classifies the brominated molecules as the R conformer.
For more detail, see: Yablon et al., J. Phys. Chem. B, in press.