Pharmacologic Treatment of Type 2 Diabetes
Which one of the following most increases insulin sensitivity in an overweight diabetic?
(Choose the one best answer.)
The Answer is E. Metformin increases insulin sensitivity much more
than sulfonylureas or insulin. This means lower insulin levels
achieve the same level of glycemic control, and may be one reason
that weight changes are less likely to be seen in diabetic patients
on metformin. Acarbose is an alpha-glucosidase inhibitor which
delays glucose absorption.
(Adapted from American Board of Family Practice question)
In discussing pharmacologic treatments of type 2 diabetes, it is important to remember the two underlying processes of insulin resistance and insulin deficiency leading to hyperglycemia. These two mechanisms are the reasons for checking both fasting and post meal glucose levels.
Oral Agents
There are seven classes of oral agents and they have different mechanisms of action. Four of the classes are secretagogues: First and second generation sulfonylureas, meglitinide, and d-Phenylalanine. Two of the classes are insulin sensitizers: biguanides and thiazolidinediones. The α-glucosidase inhibitor class delays carbohydrate absorption from the gut. Combination drug therapy can have additive effects.
The Secretagogues
All secretagogues allow the pancreas β-cells to secrete insulin in response to a glucose challenge. They are useful in patients with insulin deficiency.
Common side effects include hypoglycemia, weight gain, mild gastrointestinal complaints, and rarely skin reactions, photosensitivity, and cholestatic hepatitis. Secretagogues are contraindicated in pregnancy, and used with caution in patients with liver disease. They also should be used with caution in renal disease (except for repaglinide and nateglinide which don’t have renal dosage requirements).
There are four classes of secretagogues: first and second generation sulfonylureas, meglitinides, and d-Phenylalanine derivatives. Each secretagogue class works a little different.
First and Second Generation Sulfonylureas
Sulfonylureas bind to a sulfonylurea receptors on the β-cells which stimulate insulin secretion or sensitize the β-cells to the presence of glucose. Second generation sulfonylureas are more commonly prescribed than first generation, and have less side effects. A1C can decrease by as much as 2.3% with sulfonylureas. Most patients begin with a low dose of sulfonylurea and increase them at 1 – 2 week intervals depending on the self-monitored glucose readings and A1C results. Once daily preparations are available and are more convenient to patients.
As type 2 diabetes progresses, β-cells secrete less and less insulin and thus sulfonylureas will not be able to optimize glucose levels by themselves. Most clinicians do not discontinue them, but rather add insulin sensitizers.
Meglitinides
Meglitinides are a class of rapid-acting, short duration insulin secretagogues. The only drug of this class approved in the United States is repaglinide. It is as effective as a sulfonylurea. Repaglinide is taken before each meal and with any bedtime snacks. This class allows patients the flexibility to skip a dose if they skip a meal thus preventing hypoglycemia. Unfortunately, the patient has to take it several times a day for effectiveness. It is not contraindicated in renal insufficiency.
D-Phenylalanine Derivatives
D-Phenylalanine derivatives are a faster acting and shorter duration secretagogue than the meglitinides (rapaglinide). Nateglinide is the only member of this class. It can also be used in patients with renal insufficiency. Both nateglinide and rapaglinide can be useful in patients who are found to have optimal fasting glucose levels but high post-prandial glucose levels. Again, patients have to take it several times a day which is a drawback.
The Insulin Sensitizers
Both classes of insulin sensitizers, biguanides and thiazolidinediones, are being researched as possible therapies that delay type 2 diabetes in patients with insulin resistance, glucose intolerance (pre-diabetes), or have high risk for diabetes. These drugs are used in patients with polycystic ovarian syndrome which carries a component of insulin resistance.
Biguanides
Biguanides decrease gluconeogenesis from the liver, increases glucose uptake in muscle tissues, enhances the basal metabolic rate, and may lower food intake because of it’s gastrointestinal side effects. They do not stimulate insulin secretion from the pancreas. The exact mechanism of action is not well understood. It is indicated for patients with insulin resistance and a good consideration in those with cholesterol issues.
The only biguanide available in the United States is metformin. It can reduce the A1C by 2% and fasting glucose levels by 60 mg/dL. Metformin can decrease or stabilize patient weight, and can reduce cholesterol and triglyceride levels, and may reduce myocardial infarction risk.
Metformin should be started slow and low. A 500 mg dose started at dinner is recommended and an additional dose can be added to breakfast after a week. There are higher dose tablets and extended release forms available. Current data shows that it is most clinically effective at a dose of 2000 mg /day.
Care must be used in those with liver disease, active pulmonary or cardiac disease. Metformin is contraindicated in men who have a creatinine >1.5 mg/dL or women with a creatinine of >1.4 mg/dL. It should be withheld prior to any radiology study requiring contrast dye or if going to surgery, and restored once renal function is normal. Metformin is also contraindicated in pregnancy and breast feeding. Side effects can include flatulence, diarrhea, nausea, and a metallic taste.
Thiazolidinediones
Thiazolidinediones have an insulin sensitizing effect on the peroxisome proliferator-activated nuclear receptors in liver cells, adipose tissue, and muscle. The reduction of insulin resistance also reduces blood glucose levels. Two thiazolidinediones, rosiglitazone and pioglitazone, are approved for use in the United States. They are indicated for patients with insulin resistance.
Side effects include mild anemia, weight gain, or mild edema due to volume expansion. They can be used in patients with renal insufficiency. Liver function monitoring is recommended periodically while using these drugs, and they are contraindicated in people with liver disease who have an ALT > 2.5 times the upper limit of normal. They are contraindicated in pregnancy and may stimulate ovulation in insulin resistant anovulatory women. Thiazolidinediones are contraindicated in patients with Class III or IV New York Heart Association functional status.
Rosiglitazone has been associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes in one study. However, other published and unpublished data from long-term clinical trials of rosiglitazone provides contradictory evidence about the risks in patients treated with rosiglitazone. Patients who are taking rosiglitazone, especially those who are known to have underlying heart disease or who are at high risk of heart attack should talk to their primary care physician about this new information as they evaluate the available treatment options for their type 2 diabetes. (Link to FDA warning).
Carbohydrate Absorption Delay Agents
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors delay disaccharide and complex carbohydrate absorption in the small intestine and allow it to occur instead in the large intestine and colon. This mechanism allows improvement of glucose control. It does not have the same delay effect on lactose.
This class is excellent for patients with high 2 hour post meal hyperglycemia, and can be used in people with both insulin resistance and deficiency. They must be used with each meal to be effective. They reduce A1C by 0.5 – 1% when combined with other oral agents or insulin. Two α-glucosidase drugs are approved for use in the United States: acarbose and miglitol.
The side effects include diarrhea and flatulence. The drug should be started low and slow to prevent these side effects. Alpha-glucosidase may cause reversible liver enzyme elevation. It is contraindicated in patients with liver disease, inflammatory bowel disease, and pregnancy. They do not cause hypoglycemia by themselves, but if hypoglycemia develops in conjunction with sulfonylureas or insulin, the patient may use milk to correct their glucose level.
Selecting The Initial Agent
Once the decision that medical nutrition therapy and exercise alone are not optimizing a patient’s glucose control, the next step is to choose an appropriate oral agent. There are several choices for first-line monotherapy as per the American Diabetes Association: metformin, thiazolidinediones, or secretagogues.
Actual medication choice should incorporate:
- consideration of glucose-lowering efficacy
- contraindications
- comorbidities
- patient preference
- anticipated side effects
- any concurrent drug therapy
- frequency of dose
- cost
Most endocrinologists continue to prefer metformin as the optimal first-line agent, particularly in obese patients, and if no contraindications are present. First-line therapy with thiazolidinediones is becoming increasingly popular but some cite that there is not enough evidence based information. Thiazolidinedione studies exploring cardiovascular effects are still pending. Initial therapy with secretagogues is no longer as popular. In patients whom there appears to be a greater degree of pancreatic dysfunction as opposed to insulin resistance, secretagogue use is still appropriate. Recommendations regarding the optimal initial drug approach to this disease are always changing.
Two articles are available in the library for further review on this subject.
Combination Therapy
Most patients on monotherapy for diabetes will eventually require a second agent (50% of patients after three years of monotherapy). Using an insulin sensitizing medication along with a secretagogue, or two insulin resistance drugs, are good choices. In some patients, first line combination therapy may be considered sooner: 1. If the patient has an A1C >9% before MNT has been instituted 2. A1C >8% after MNT has been tried. Pharmaceutical companies are now formulating combination of different diabetes drugs into one pill (metformin – glyburide) which may reduce pill burden, reduce co-payment cost to the patient, and make adherence easier. One disadvantage is less ability in making small titrating adjustments.
Triple therapy is increasingly more common. Consideration should be given as to whether a patient on triple oral therapy should actually be on insulin.
Insulin
In type 1 diabetes, insulin therapy is mandatory. In type 2 diabetes, progressive insulin deficiency makes insulin a useful therapeutic tool. Insulin is the most potent therapy for diabetes.
Goals of Therapy
The normal pattern of insulin levels throughout the day is illustrated in the chart below.
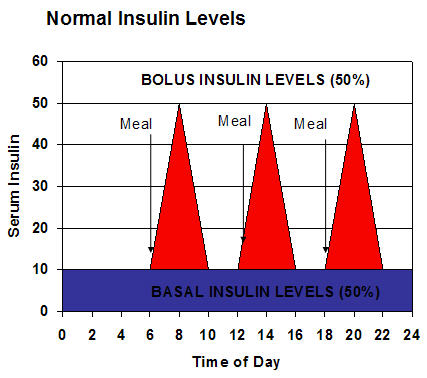
The pancreas is constantly secreting basal levels of insulin which provides 50% of the body’s requirement. After a meal, the pancreatic β-cells secretes insulin in response to meals known as bolus levels, which supplies the body’s other 50% requirement. Insulin therapy attempts to simulate this normal pattern. In most diabetic patients, multiple daily doses are required to strike the right balance between glycemic control and avoiding hypoglycemia. Studies have found that physicians should probably begin using insulin on patients earlier than they do, and that about 50% of type 2 diabetics require insulin to keep their A1C <7%.
Types of Insulin
There are several types of insulin available, and their use is based on the type of insulin therapy and the onset of action required. Premixed types combine basal and background insulin.
Insulin Uses, Types, and Onset of Actions |
||
Use |
Types |
Examples |
Bolus Insulin
|
|
|
|
|
|
Basal Insulin
|
|
|
|
|
|
|
|
|
Premixed |
|
|
|
|
|
Bolus Insulin
Bolus insulin is designed to cover a meal and is either rapid acting or short acting. Rapid acting insulin is usually recommended for most patients. They are taken within 15 minutes before a meal and are cleared from the body in 2 – 4 hours. Glucose monitoring 2 hours after a meal allows for adjustments in the dose. Postprandial glucose levels tend to be lower with rapid acting than with short acting insulin. Rapid acting insulin allows for flexibility and patients can exercise at any time. Rapid acting insulin is a good choice for those who don’t snack throughout the day, while short acting insulin may be better for patients who frequently delay eating after an injection or eat throughout the day. Bolus insulin is often used in conjunction with basal insulin dosing.
Basal Insulin
Basal insulin covers the baseline insulin needs of the body and is usually intermediate acting, extended intermediate acting, or long acting. The two intermediate acting insulins are NPH and Lente. They have a short duration of action and act quickly. Ultralente acts somewhat longer than NPH and is therefore known as extended intermediate acting insulin. Glargine (brand name is Lantus) is a long acting insulin analogue. It is formulated for delayed absorption over 24 hours with no peak levels, can be administered once a day, and has a lower risk of hypoglycemia. Glargine can not be mixed with other insulin types, and is usually used in conjunction with bolus insulin.
Insulin Types |
||||
Type of Insulin |
Examples |
Onset of Action |
Peak of Action |
Duration of Action |
Rapid-acting |
Humalog (lispro) |
15 minutes |
30-90 minutes |
3-5 hours |
NovoLog (aspart) |
15 minutes |
40-50 minutes |
3-5 hours |
|
Short-acting (Regular) |
Humulin R |
30-60 minutes |
50-120 minutes |
5-8 hours |
Intermediate-acting (NPH) |
Humulin N |
1-3 hours |
8 hours |
20 hours |
Humulin L |
1-2.5 hours |
7-15 hours |
18-24 |
|
Intermediate- and short-acting mixtures |
Humulin 50/50 |
The onset, peak, and duration of action of these mixtures would reflect a composite of the intermediate and short- or rapid-acting components, with one peak of action. |
||
Long-acting |
Ultralente |
4-8 hours |
8-12 hours |
36 hours |
Lantus (glargine) |
1 hour |
none |
24 hours |
|
Adapted from Federal Drug Administration Source, 2006 |
||||
Insulin Regimens
Basic Insulin Regimens
For most patients initiating insulin, they should probably start with basic regimens. As their knowledge base and comfort grows, they can be changed to advanced regimens. Here are some recommendations of basic insulin regimens from the American Academy of Family Medicine and the American Diabetes Association:
BASIC INSULIN REGIMENS |
||||
CANDIDATES - Use for patients with 2 or more of the following:
|
||||
REGIMEN |
COMMENTS |
AM |
PM |
BEDTIME |
RAPID ACTING + NPH |
Use for pts who choose NOT to snack; consider when post-meal hyperglycemia is present |
RA |
RA |
NPH |
RAPID ACTING + NPH PREMIX PEN |
Use premixed pen as easier way to start insulin or if unable to measure / mix; Use for pts who choose NOT to snack; consider when post-meal hyperglycemia is present |
RA |
RA |
_ |
NPH + REGULAR |
Use for patients who choose to snack; May be cheaper than rapid acting + NPH |
Regular |
Regular |
NPH |
NPH + REGULAR PREMIX PEN |
Use premixed pen as easier way to start insulin or if unable to measure / mix; Use for patients who choose to snack; may be cheaper than rapid acting + NPH |
Regular |
Regular |
_ |
NPH WITH ORAL AGENTS |
Use for type 2 DM with high fasting glucose (with or without subsequent elevated pre-meal glucose); continuing biguanides or TZD to add glycemic control |
ORAL AGENTS |
NPH |
|
GLARGINE WITH ORAL AGENTS |
Use for type 2 DM with high fasting glucose (with or without subsequent elevated pre-meal glucose); continuing biguanides or TZD to add glycemic control |
ORAL AGENTS |
GLARGINE |
|
Adapted from AAFP and ADA recommendations |
||||
Premixed insulin with NPH and a rapid acting component is more expensive but provides better post meal glucose control. The usual starting dose is 0..3 – 0.5 U / kg / day generally increasing to 0.7 – 1.2 U / kg / day.
For single night time dose of Glargine, the initial dose is 0.1 – 0.2 U / kg / day. The manufacturer recommends an initial dose of 10 U. The target is a morning fasting glucose of <140 mg/dL. The dose can be increased by 2 – 5 units every 4 – 7 days until the morning fasting glucose has reached target.
Advanced Insulin Therapy
Once the patient is comfortable with a basic insulin regimen and the daily doses is known, most individuals require more aggressive therapy. Basic regimens are inflexible and can increase the risk of hypoglycemia. Advanced regimens are more flexible for a patient’s life demands (ex. exercise regimen changes, work schedule demands, meal intake variability). It requires both an increased frequency of insulin administration and self monitored glucose levels. Patient education is critical and they must understand the effects of insulin, carbohydrate intake, insulin injection administration, and exercise. Most advanced regimens use rapid acting insulin and a long acting insulin. The following is a recommendation also based on the ADA and AAFP:
ADVANCED INSULIN REGIMENS |
||||
REGIMEN |
AM |
NOON |
PM |
BEDTIME |
RAPID ACTING + |
RA |
RA only |
RA |
Small dose of NPH for some |
RAPID ACTING + |
RA |
RA |
RA |
GLARGINE |
Regular insulin can be substituted for patients who snack without bolus coverage or if there is a cost issue for patients. |
||||
The initial insulin dosage for advanced insulin therapy is on the order of 0.4 to 0.5 U / kg / day for a patient naïve to insulin. Many patients will require more over time on the order of 1 – 2 U / kg / day. Typically half of the total will go to the bolus dose and half to the basal dose.
A 200 pound man who is naïve to insulin is started on advanced insulin therapy of rapid acting insulin and glargine. Assume you start him on dosage of 0.5 U / kg / day. How many units of glargine will he receive at bedtime and how many units of rapid acting insulin will he get at breakfast, lunch, and dinner?
(Choose the one best answer.)
The answer is B. In this sample case of a 200 pound man who has never taken insulin prior:
Step One) 200 lb. man ÷ 2.2 = 90.9 kg man
Step Two) Initial dose of advanced insulin regimen for insulin naive patient: 0.5 U/kg/day
90.9 kg X 0.5 U/kg = 45.5 U / day -> can round to 46 U/day
Step Three) 50% goes to basal dose = 23 U of glargine at night
Step Four) 50% goes to bolus doses of rapid acting insulin with meals = 23 U of rapid acting insulin / day
Step Five) The 23 U of rapid acting insulin would be distributed over the morning (breakfast), noon (lunch), and evening (dinner) dose. Thus about 7.5 - 7.7 U/meal/day.
In reality, most patients have variable carbohydrate intake amounts with meals. Using the carbohydrate counting method comes in handy to better distribute the bolus amount of rapid acting insulin with each meal.
Insulin Adjustments
Insulin dosage should always be adjusted for hypoglycemia first. If all self monitored glucose levels are greater than 200 mg/dL, then the total daily dose of insulin should be increased by 0.1 U/kg. Fasting and pre-meal glucose levels will help adjust basal insulin doses. 2 hour post meal glucose levels will help adjust bolus insulin doses.
- If current dose is ≤10 U, dose can be increased by 1 U if glucose levels are high or lowered by 1 U if glucose levels are low
- If current dose is >10U, the dose can be increased by 10% if glucose levels are high or lowered by 10% if glucose levels are low
The target glucose level for rapid-acting insulin is achieved when the 2 hour post meal glucose level is within 20 – 40 mg of the pre meal glucose level. Patients can also be taught to administer insulin sliding scales in the event of unexpected high glucose levels.
Alternate Insulin Administration Methods
Insulin pumps may be useful for some patients as they deliver rapid acting insulin on a continuous basis as a basal dose. The patient then pushes a button to deliver bolus doses with meals.
Inhaled insulin is a new dry powder method that will be available soon as it has been approved by the FDA. Although it may be more acceptable to administer than injected insulin, it is subject to more variability in patient skill of administration, is less flexible in dosing, may still require injected basal insulin, are contraindicated in patients with lung disease, and its long term effects on the lungs are unknown.
More information can be found at this link:
http://www.fda.gov/bbs/topics/news/2006/NEW01304.html
Side Effects of Injected Insulin
Lipodystrophy can happen at sites of injection, with lipohypertrophy occurring more often in men and lipoatrophy occurring more commonly in women. Changing injection sites can prevent this side effect.
Local skin reactions such as itching, redness, and discomfort may occur. Most reactions resolve with desensitization over 6 weeks. Antihistamines can be used in the interim.
Systemic reactions are rare but allergies can develop. They can vary from urticaria to anaphylaxis. It is more common in patients with penicillin allergies or atopic dermatitis. It also occurs more frequently in those patients using insulin intermittently. Desensitization therapy is now available.
New Medications
Incretin Mimetic Agents
Exenatide is the first drug in a new class known as the incretin mimetic agents. It improves glucose control by mimicking the effects of glucagon-like peptide-1, a natural mammalian incretin hormone secreted during food intake. Exenatide was approved by the U.S. Food and Drug Administration for the treatment of type 2 diabetes in conjunction with metformin and/or sulfonylurea. The recommended dosage is 5 mug to 10 mug twice daily subcutaneously before breakfast and dinner. In randomized, placebo-controlled, 30-week clinical studies, exenatide improved glycemic control and promoted weight loss of up to 2.8 kg. The most common adverse effects were nausea, vomiting, diarrhea, and dose-dependent hypoglycemia. Patients should be closely monitored for hypoglycemia, especially when exenatide is added to sulfonylurea therapy. Overall, exenatide provides a treatment option for patients with type 2 diabetes who fail to obtain glycemic control while on a maximum dose of metformin and/or sulfonylurea therapy. It is also an alternative therapy for those patients who cannot tolerate other antidiabetic drugs. (Cardiol Rev. 2006 Abstract)