Leighton Group Publications
85. Design and 22-step synthesis of highly potent D-ring modified and linker-equipped analogs of spongistatin 1 Suen, L. M.; Tekle-Smith, M. A.; Williamson, K. S.; Infantine, J. R.; Reznik, S. K.; Tanis, P. S.; Casselman, T. D.; Sackett, D. L.; Leighton, J. L. Nature Communications 2018, 9, 4710
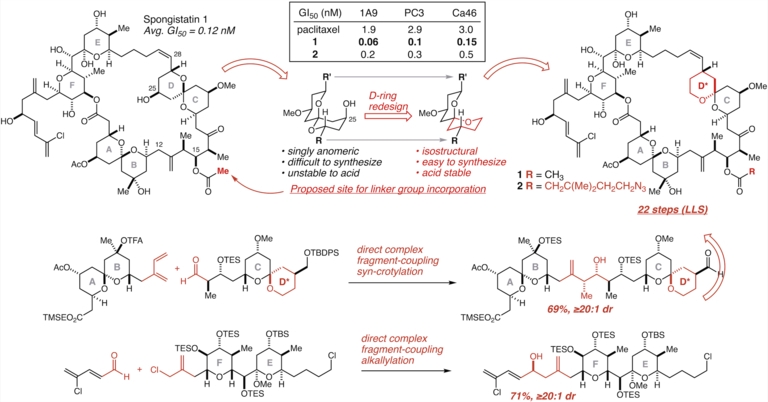
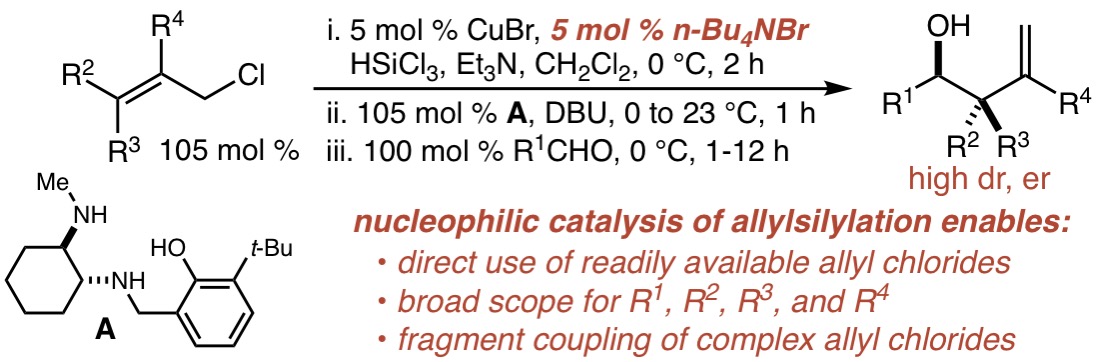
84. Direct, Mild, and General n-Bu4NBr-Catalyzed Aldehyde Allylsilylation with Allyl Chlorides Tekle-Smith, M. A.; Williamson, K. S.; Hughes, I. F.; Leighton, J. L. Org. Lett. 2017, 19, 6024–6027.
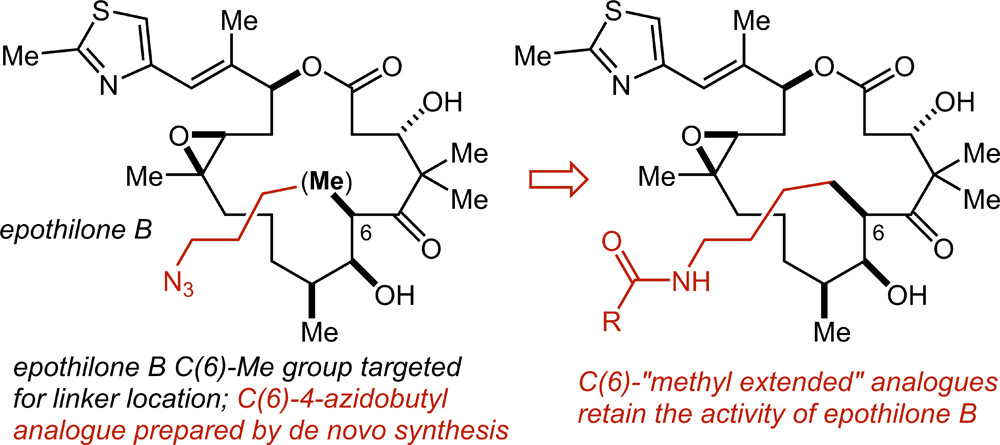
83. Synthesis and Evaluation of a Linkable Functional Group-Equipped Analogue of the Epothilones Foley, C. N.; Chen, L.-A.; Sackett, D. L.; Leighton, J. L. ACS Med. Chem. Lett. 2017, 8(7), 701-704.
Article featured on the cover of ACS Medicinal Chemistry Letters
82. Silane and Germane Molecular Electronics Su, T. A.; Li, H.; Klausen, R. S.; Kim, N. T.; Neupane, M; Leighton, J. L.; Steigerwald, M. L.; Venkataraman, L.; Nuckolls, C. Acc. Chem. Res. 2017, 50, 1088–1095.
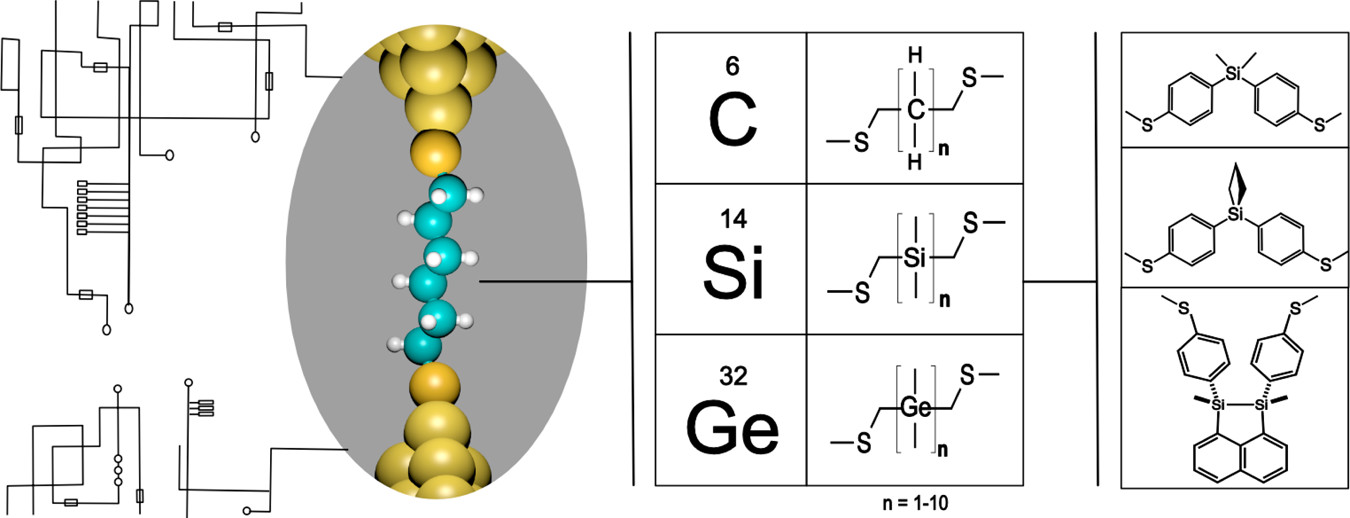
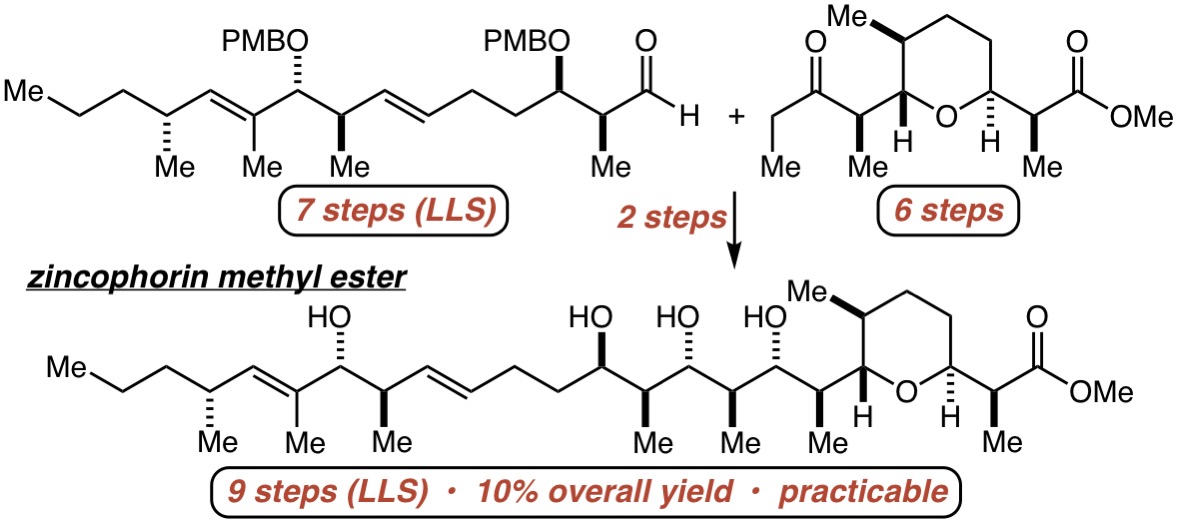
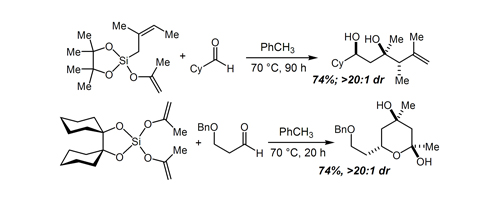
81. Evolution of an Efficient and Scalable Nine-Step (Longest Linear Sequence) Synthesis of Zincophorin Methyl Ester Chen, L.-A.; Ashley, M. A.; Leighton, J. L. J. Am. Chem. Soc. 2017, 139, 4568–4573.
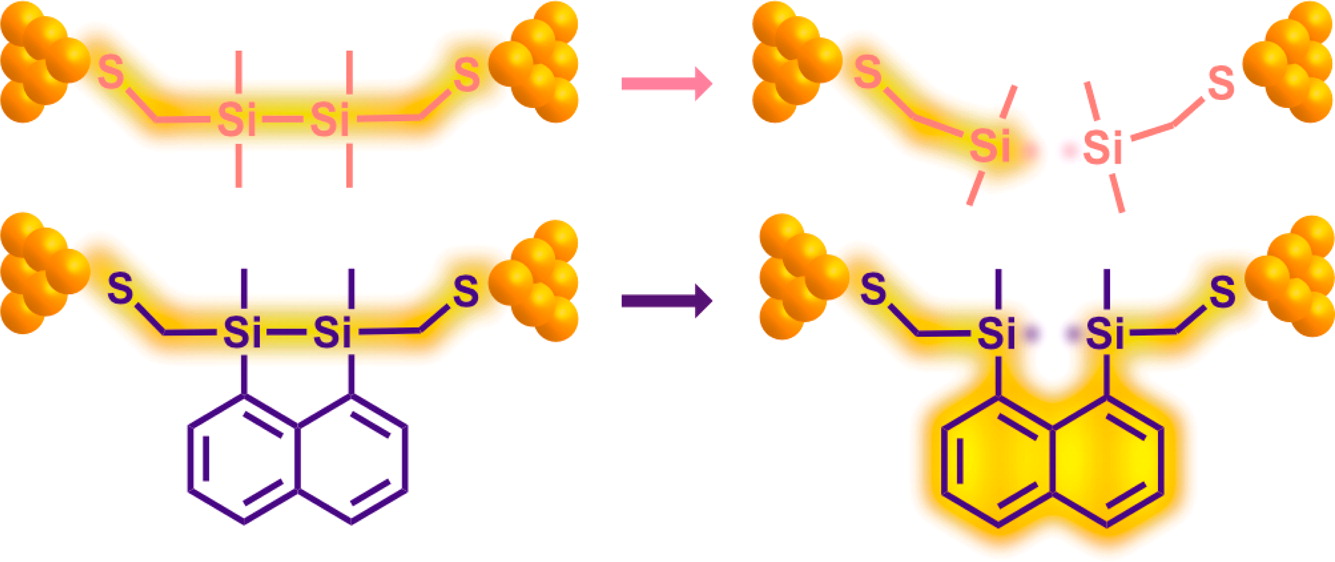
80. Mechanism for Si-Si Bond Rupture in Single Molecule Junctions Li, H.; Kim, N. T.; Su, T. A.; Steigerwald, M. L.; Nuckolls, C.; Darancet, P.; Leighton, J. L.; Venkataraman, L. J. Am. Chem. Soc. 2016, 138, 16159–16164.
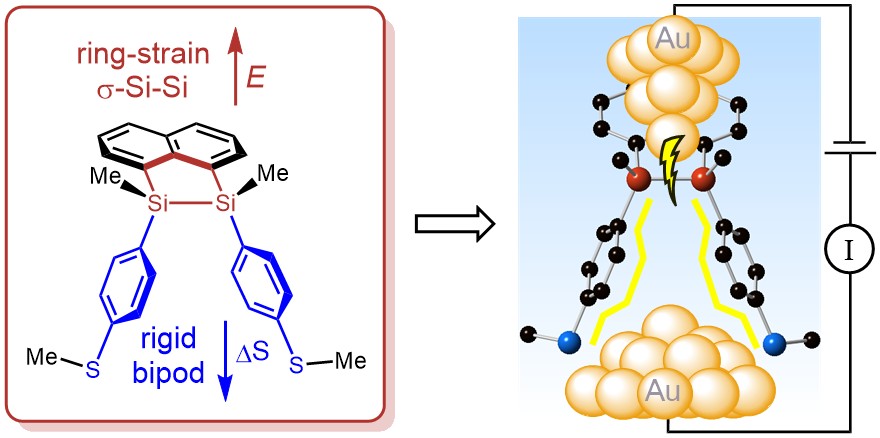
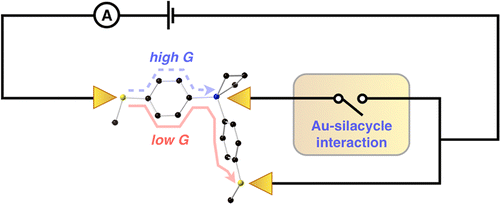
79. High-Conductance Pathways in Ring-Strained Disilanes by Way of Direct σ-Si-Si to Au Coordination Kim, N. T.; Li, H.; Venkataraman, L.; Leighton, J. L. J. Am. Chem. Soc. 2016, 138, 11505–11508.
78. A Highly Stereoselective, Efficient, and Scalable Synthesis of the C(1)-C(9) Fragment of the Epothilones Foley, C. N.; Leighton, J. L. Org. Lett. 2015, 17, 5858–5861.
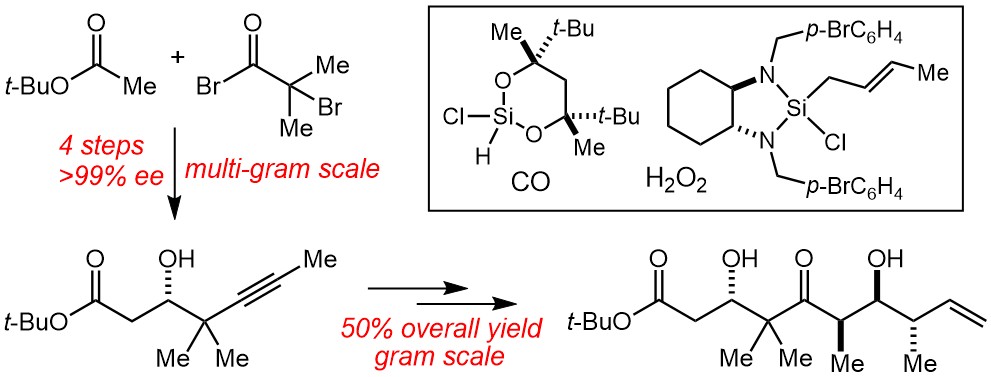
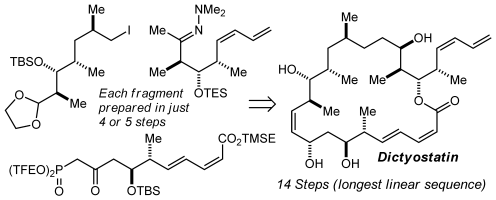
77. A “Methyl Extension” Strategy for Polyketide Natural Product Linker Site Validation and Its Application to Dictyostatin Ho, S.; Sackett, D. L.; Leighton, J. L. J. Am. Chem. Soc. 2015, 137, 14047–14050.
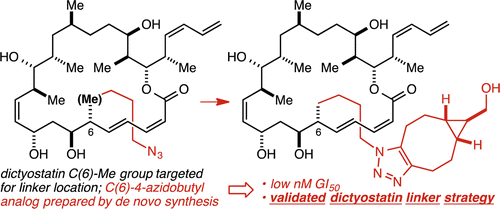
76. Design, Development, Mechanistic Elucidation, and Rational Optimization of a Tandem Ireland Claisen/Cope Rearrangement Reaction for Rapid Access to the (Iso)Cyclocitrinol Core Plummer, C. W.; Wei, C. S.; Yozwiak, C. E.; Soheili, A.; Smithback, S. O.; Leighton, J. L. J. Am. Chem. Soc. 2014, 136, 9878-9881.
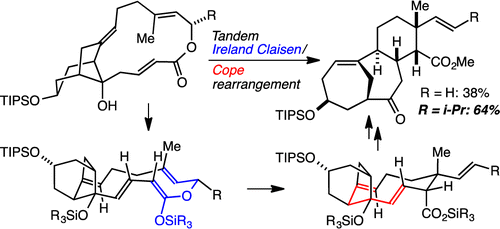
75. Beyond the Roche ester: a new approach to polypropionate stereotriad synthesis. Foley, C. N.; Leighton, J. L. Org. Lett. 2014, 16, 1180-1183.
74. Silicon Ring Strain Creates High-Conductance Pathways in Single-Molecule Circuits. Su, T. A.; Widawsky, J. R.; Li, H.; Klausen, R. S.; Leighton, J. L.; Steigerwald, M. L.; Venkataraman, L.; Nuckolls, C. J. Am. Chem. Soc. 2013, 135, 18331-18334.
73. Exploiting Pseudo C2-Symmetry for an Efficient Synthesis of the F-Ring of the Spongistatins Tanis, P. S.; Infantine, J. R.; Leighton, J. L. Org. Lett. 2013, 15, 5464-5467.
72. A Highly Step-Economical Synthesis of Dictyostatin Ho, S.; Bucher, C.; Leighton, J. L. Angew. Chem. Int. Ed. 2013, 52, 6757-6761.
71. A New and More Powerfully Activating Diamine for Practical and Scalable Enantioselective Aldehyde Crotylsilylation Reactions Suen, L. M.; Steigerwald, M. L.; Leighton, J. L. Chem. Sci. 2013, 4, 2413-2417.
70. Toward a More Step-Economical and Scalable Synthesis of Spongistatin 1 to Facilitate Cancer Drug Development Efforts Reznik, S. K.; Leighton, J. L. Chem. Sci. 2013, 4, 1497-1501.
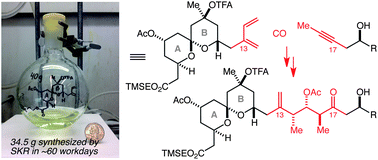
69. Complex Fragment Coupling by Crotylation: A Powerful Tool for Polyketide Natural Product Synthesis Reznik, S. K.; Marcus, B. S.; Leighton, J. L. Chem. Sci. 2012, 3, 3326-3330.
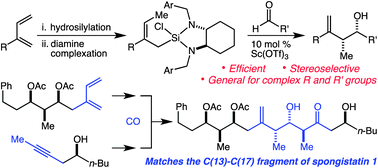
68. An "Aprotic" Tamao Oxidation/Syn-Selective Tautomerization Reaction for the Efficient Synthesis of the C(1)–(9) Fragment of Fludelone Harrison, T. J.; Rabbat, P. M. A.; Leighton, J. L. Org. Lett. 2012, 14, 4890-4893.
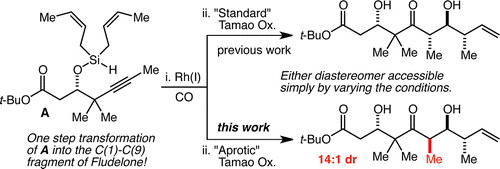
67. Direct and highly regioselective and enantioselective allylation of Β–diketones Chalifoux, W. A.; Reznik, S. K.; Leighton, J. L. Nature 2012, 487, 86-89.
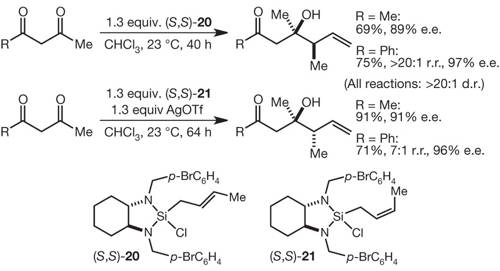
66. A Tandem Cross-Metathesis/Semipinacol Rearrangement Reaction Plummer, C. W.; Soheili, A.; Leighton, J. L. Org. Lett. 2012, 14, 2462-2464.
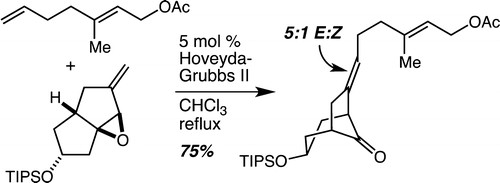
65. Direct and Highly Enantioselective Iso–Pictet–Spengler Reactions with α–Ketoamides: Access to Underexplored Indole Core Structures Schönherr, H.; Leighton, J. L. Org. Lett. 2012, 14, 2610-2613.
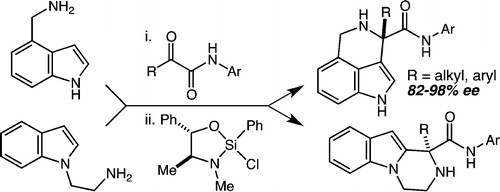
64. A New Synthesis of Pyrrolidines by Way of an Enantioselective Mannich/Diastereoselective Hydroamination Reaction Sequence Baxter Vu, J. M.; Leighton, J. L. Org. Lett. 2011, 13, 4056-4059.
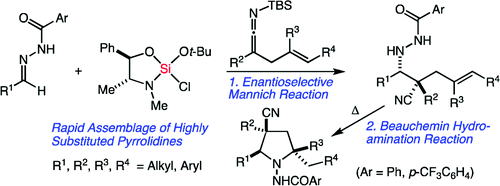
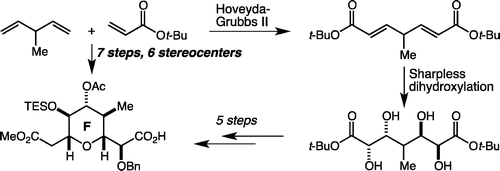
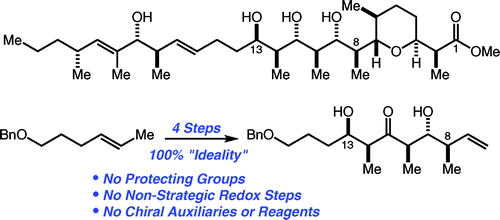
63. Toward More ”Ideal” Polyketide Natural Product Synthesis: A Step-Economical Synthesis of Zincophorin Methyl Ester Harrison, T. J.; Ho, S.; Leighton, J. L. J. Am. Chem. Soc. 2011, 133, 7308-7311.
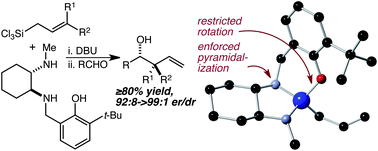
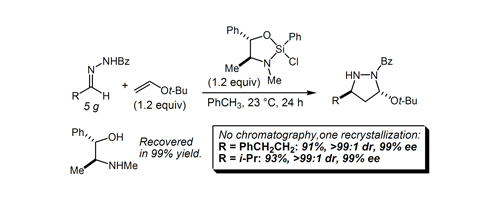
62. A More Comprehensive and Highly Practical Solution to Enantioselective Aldehyde Crotylation Kim, H.; Ho, S.; Leighton, J. L. J. Am. Chem. Soc. 2011, 133, 6517-6520.
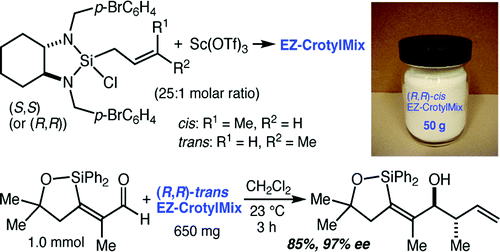
61. Highly Enantioselective Mannich Reactions with α–Aryl Silyl Ketene Acetals and Imines Notte, G. T.; Baxter Vu, J. M.; Leighton, J. L. Org. Lett. 2011, 13, 816-818.
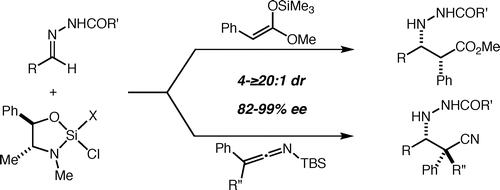
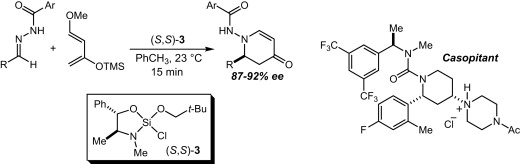
60. Enantioselective (Formal) Aza-Diels-Alder Reactions with Non-Danishefsky-Type Dienes Tambar, U. K.; Lee, S. K.; Leighton, J. L. J. Am. Chem. Soc. 2010, 132, 10248-10250.
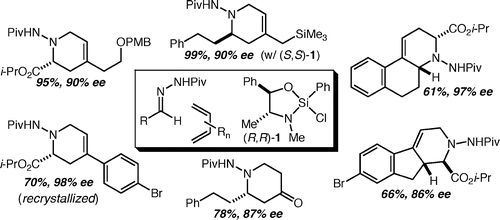
59. Highly enantioselective formal aza-Diels-Alder reactions with acylhydrazones and Danishefsky's diene promoted by a silicon Lewis acid Lee, S. K.; Tambar, U. K.; Perl, N. R.; Leighton, J. L. Tetrahedron 2010, 66, 4769-4774.
Invited article for the Brian Stoltz Tetrahedron Young Investigator Award special issue.
58. Asymmetric Allylation, Crotylation, and Cinnamylation of N-Heteroaryl Hydrazones Feske, M. I.; Buitrago Santanilla, A.; Leighton, J. L. Org. Lett. 2010, 12, 688-691.
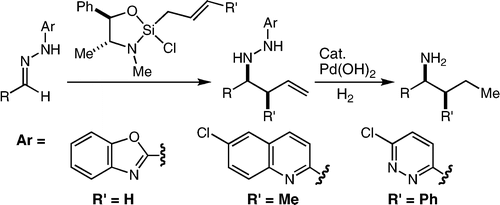
57. Tandem Asymmetric Aza-Darzens/Ring-Opening Reactions: Dual Functionality from the Silane Lewis Acid Valdez, S. C.; Leighton, J. L. J. Am. Chem. Soc. 2009, 131, 14638-14639.
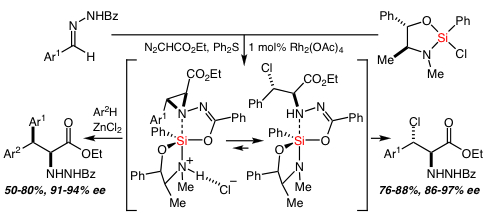
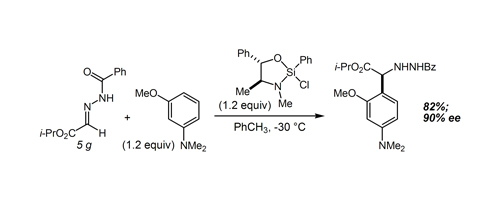
56. Highly Enantioselective Pictet-Spengler Reactions with α–Ketoamide-Derived Ketimines: Access to an Unusual Class of Quaternary α–Amino Amides Bou-Hamdan, F. R.; Leighton, J. L. Angew. Chem. Int. Ed. 2009, 48, 2403-2406.
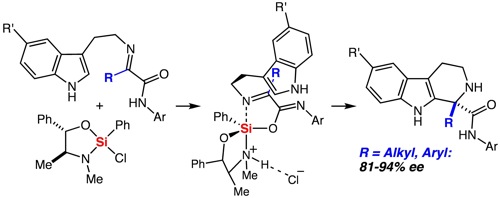
55. Tandem Silylformylation-Crotylsilylation/Tamao Oxidation of Internal Alkynes: A Remarkable Example of Generating Complexity from Simplicity Spletstoser, J. T.; Zacuto, M. J.; Leighton, J. L. Org. Lett. 2008, 10, 5593-5596.
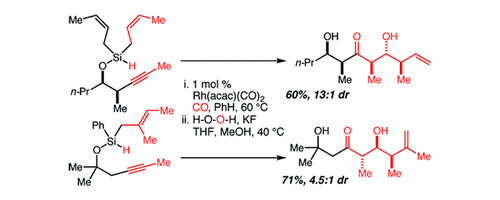
54. An Efficient Asymmetric Synthesis of Manzacidin C Tran, K.; Lombardi, P. J.; Leighton, J. L. Org. Lett. 2008, 10, 3165-3167.
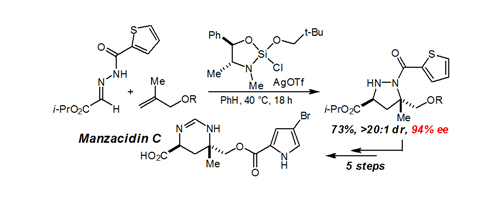
53. A New Silicon Lewis Acid for Highly Enantioselective Mannich Reactions of Aliphatic Ketone-Derived Hydrazones Notte, G. T.; Leighton, J. L. J. Am. Chem. Soc. 2008, 130, 6676-6677.
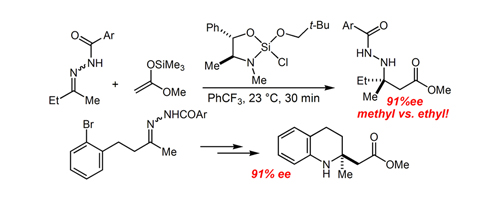
52. Allylsilane Vinylarene Cross-Metathesis Enables a Powerful New Approach to Enantioselective Imine Allylation Huber, J. D.; Perl, N. R.; Leighton, J. L. Angew. Chem. Int. Ed. 2008, 47, 3037-3039.
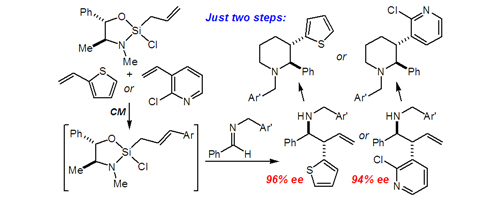
51. Highly Enantioselective Imine Cinnamylation with a Remarkable Diastereochemical Switch Huber, J. D.; Leighton, J. L. J. Am. Chem. Soc. 2007, 129, 14552-14553.
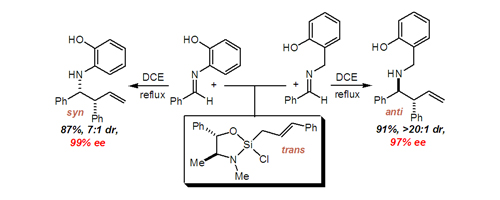
50. Enantioselective Imidazole-Directed Allylation of Aldimines and Ketimines Perl N. R.; Leighton, J. L. Org. Lett. 2007, 9, 3699-3701.
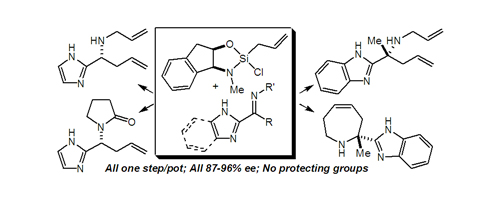
49. A Modified Approach to the Phomoidrides: Synthesis of a Late-Stage Intermediate Containing a Key Carbon Quaternary Center Castagner, B.; Leighton, J. L. Tetrahedron 2007, 63, 5895-5902.
Invited article for the Tetrahedron 50th Anniversary Symposium-in-Print special issue.
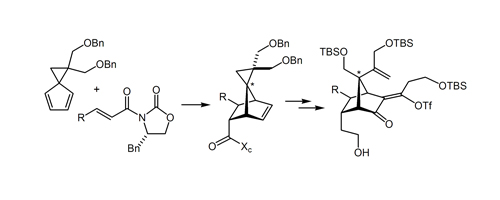
48. Phenol-Directed Enantioselective Allylation of Aldimines and Ketimines Rabbat, P. M. A.; Valdez, S. C.; Leighton, J. L. Org. Lett. 2006, 8, 6119-6121.
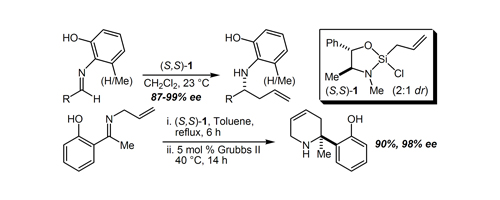
47. A Simple, Efficient, and Highly Enantioselective Synthesis of MS-153 Employing a Chiral Silane Lewis Acid-Promoted Acylhydrazone-Enol Ether [3+2] Cycloaddition Tran, K.; Leighton, J. L. Adv. Synth. Catal. 2006, 348, 2431-2436.
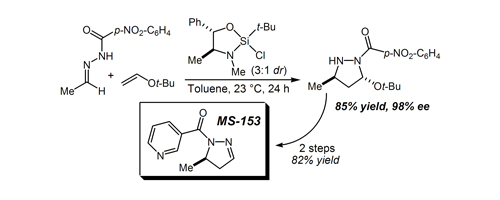
46. Strained Silacycle-Catalyzed Asymmetric Diels-Alder Cycloadditions: The First Highly Enantioselective Silicon Lewis Acid Catalyst Kubota, K.; Hamblett, C. L.; Wang, X.; Leighton, J. L. Tetrahedron 2006, 62, 11397-11401.
Invited article for the David MacMillan Tetrahedron Young Investigator Awardspecial issue.
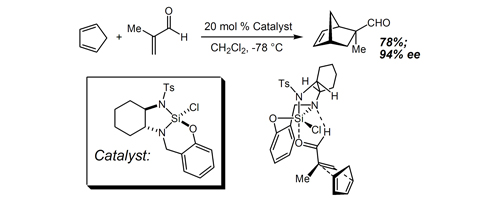
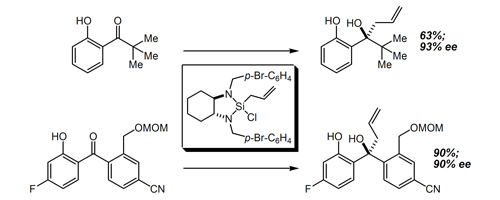
45. A Method for the Enantioselective Allylation and Crotylation of Sterically Hindered and Functionalized Aryl Ketones: Convenient and Unprecedented Access to Unusual Tertiary Carbinol Structures Burns, N. Z.; Hackman, B. M.; Ng, P. Y.; Powelson, I. A.; Leighton, J. L. Angew. Chem. Int. Ed. 2006, 45, 3811-3813.
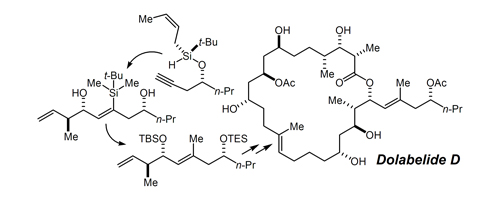
44. Total Synthesis of Dolabelide D" Park, P. K.; O'Malley, S. J.; Schmidt, D. R.; Leighton, J. L. J. Am. Chem. Soc. 2006, 128, 2796-2797.
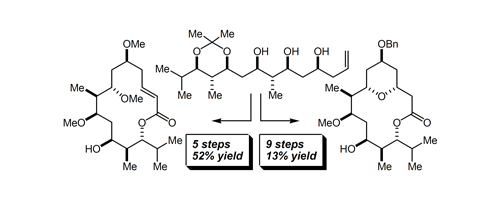
43. Divergent Synthesis of Complex Polyketide-Like Macrolides from a Simple Polyol Fragment Zacuto, M. J.; Leighton, J. L. Org. Lett. 2005, 7, 5525-5527.
42. Tandem Aldol-Allylation and Aldol-Aldol Reactions with Ketone-Derived Enolsilanes: Highly Diastereoselective Single-Step Synthesis of Complex Tertiary Carbinols Wang, X.; Meng Q.; Perl N. R.; Xu Y.; Leighton, J. L. J. Am. Chem. Soc. 2005, 127, 12806-12807.
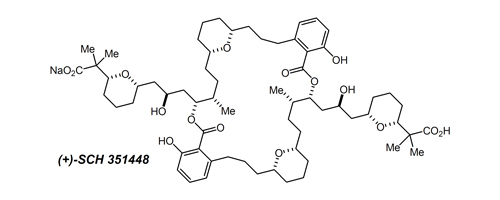
41. Efficient Asymmetric Synthesis of (+)-SCH 351448 Bolshakov, S.; Leighton, J. L. Org. Lett. 2005, 7, 3809-3812.
40. A Simple and General Chiral Silicon Lewis Acid for Asymmetric Synthesis: Highly Enantioselective [3+2] Acylhydrazone-Enol Ether Cycloadditions Shirakawa, S.; Lombardi, P. J.; Leighton, J. L. J. Am. Chem. Soc. 2005, 127, 9974-9975.
39. Enantioselective Friedel-Crafts Alkylations with Benzoylhydrazones Promoted by a Simple Strained Silacycle Reagent Shirakawa, S.; Berger, R.; Leighton, J. L. J. Am. Chem. Soc. 2005, 127, 2858-2859.
38. Origins of Stereoselectivity in Strain-Release Allylations Zhang, X.; Houk, K. N.; Leighton, J. L. Angew. Chem. Int. Ed. 2005, 44, 938-941.
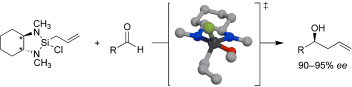
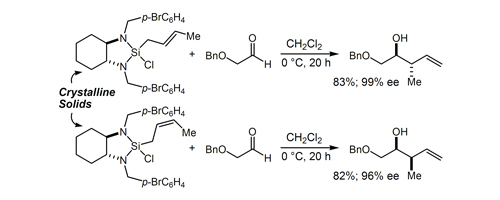
37. Highly Diastereo- and Enantioselective Reagents for Aldehyde Crotylation Hackman, B. M.; Lombardi, P. J.; Leighton, J. L. Org. Lett. 2004, 6, 4375-4377.
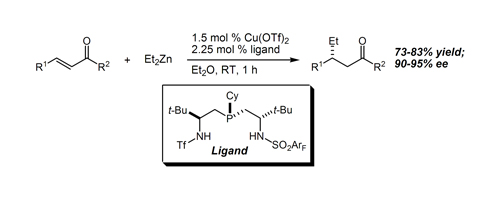
36. Enantioselective Cu-Catalyzed Conjugate Addition of Diethylzinc to Acyclic Aliphatic Enones Duncan, A. P.; Leighton, J. L. Org. Lett. 2004, 6, 4117-4119.
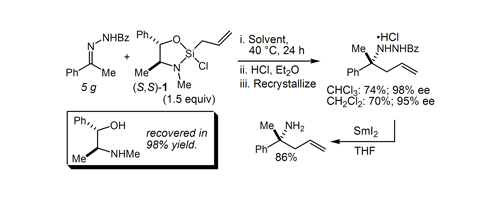
35. Enantioselective Allylation of Ketone-Derived Benzoylhydrazones: Practical Synthesis of Tertiary Carbinamines Berger, R.; Duff, K; Leighton, J. L. J. Am. Chem. Soc. 2004, 126, 5686-5687.
34. An Approach to the Synthesis of Dolabelides A and B: Fragment Synthesis by Tandem Silylformylation-Crotylsilylation Schmidt, D. R.; Park, P. K.; Leighton, J. L. Org. Lett. 2003, 5, 3535-3537.
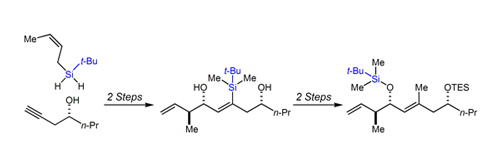
33. Tandem Silylformylation-Allyl(crotyl)silylation: A New Approach to Polyketide Synthesis Zacuto, M. J.; O' Malley, S. L.; Leighton, J. L. Tetrahedron 2003, 59, 8889-8900.
Invited article for the New Synthetic Methods VII special issue.
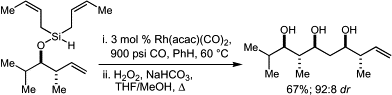
32. Highly Enantioselective Cu-Catalyzed Conjugate Addition of Alkylzinc Reagents to Cyclic Enones at Ambient Temperature Krauss, I. J.; Leighton, J. L. Org. Lett. 2003, 5, 3201-3203.
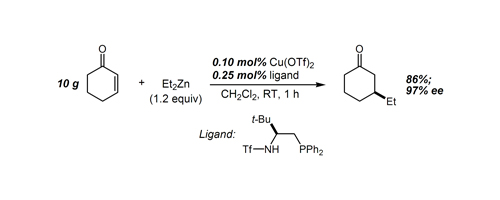
31. Toward a Versatile Allylation Reagent: Practical, Enantioselective Allylation of Acylhydazones Using Strained Silacycles Berger, R.; Rabbat, P. M. A.; Leighton, J. L. J. Am. Chem. Soc. 2003, 125, 9596-9597.
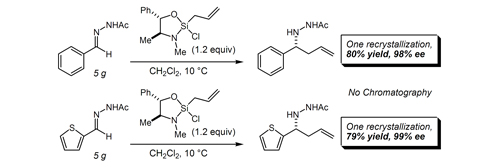
30. An Approach to the Synthesis of the Phomoidrides Bio, M. M.; Leighton, J. L. J. Org. Chem. 2003, 68, 1693-1700.
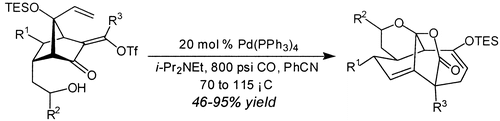
29. A Highly Enantioselective and Practical Reagent for the Allylation of Aldehydes Zhang, X.; Houk, K. N.; Leighton, J. L. Angew. Chem. Int. Ed. 2003, 42, 946-948.
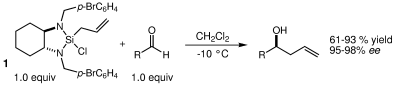
28. Catalytic Asymmetric Silane Alcoholysis: Practical Access to Chiral Silanes Schmidt, D. R.; O' Malley, S. L.; Leighton, J. L. J. Am. Chem. Soc. 2003, 125, 1190-1191.
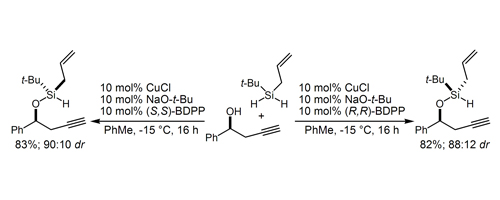
27. Strained Silacylces in Organic Synthesis: The Tandem Aldol-Allylation Reaction Wang, X.; Meng, Q.; Nation, A. J.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 10672-10673.
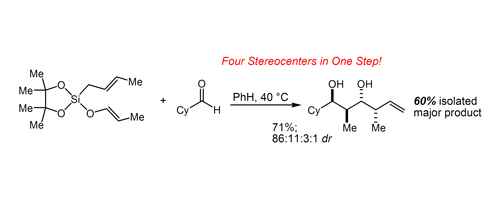
26. Strained Silacylces in Organic Synthesis: A New Reagent for the Enantioselective Allylation of Aldehydes Kinnaird, J. W. A.; Ng, P. Y.; Kubota, K.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 7920-2921.
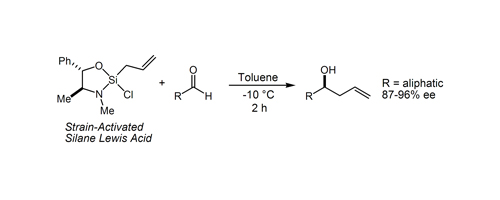
25. Tandem Intramolecular Silylformylation-Crotosilylation: Highly Efficient Synthesis of Polyketide Fragments Zacuto, M. J.; O'Malley, S. J.; Leighton, J. L. J. Am. Chem. Soc. 2002, 124, 7890-7891.
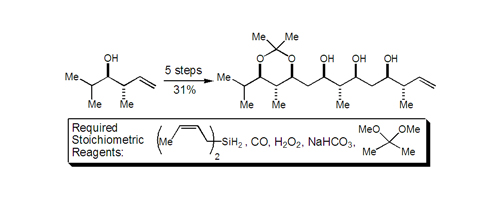
24. Highly Regioselective and Diastereoselective Directed Hydroformylation of Allylic Ethers: A New Approach to Propionate Aldol Synthesis Krauss, I. J.; Wang, C. C.-Y.; Leighton, J. L. J. Am. Chem. Soc. 2001, 123, 11514-11515.
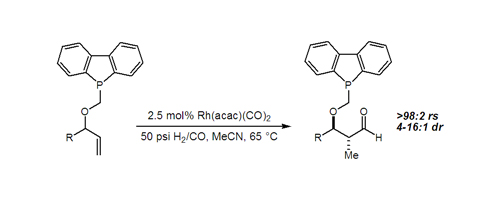
23. Tandem Intramolecular Alkyne Silylformylation-Allylsilylation: A Case of Remote 1,5-Asymmetric Induction O'Malley, S. J.; Leighton, J. L. Angew. Chem. Int. Ed. 2001, 40, 2915-2917.
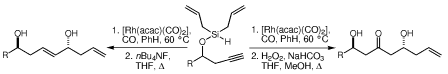
22. Formal Total Synthesis of Mycoticin A Dreher, S. D.; Leighton, J. L. J. Am. Chem. Soc. 2001, 123, 341-342.
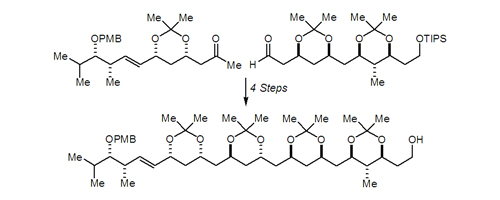
21. Total Synthesis of Leucascandrolide A Hornberger, K. R.; Hamblett, C. L.; Leighton, J. L. J. Am. Chem. Soc. 2000, 122, 12894-12895.
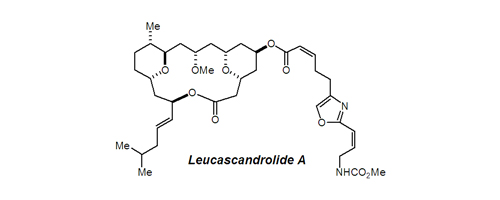
20. Rhodium-Catalyzed Formylation of Organomercurials: Application to Efficient Polyol Synthesis Sarraf, S. T.; Leighton, J. L. Org. Lett. 2000, 2, 3205-3208.
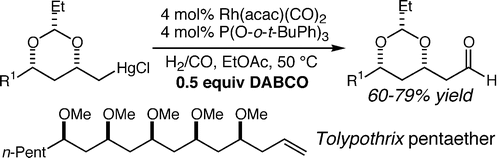
19. Yb(OTf)3-Catalyzed Oxymercuration of Homoallylic Alcohol-Derived Hemiacetals and Hemiketals Dreher, S. D.; Hornberger, K. R.; Sarraf, S. T.; Leighton, J. L. Org. Lett. 2000, 2, 3197-3199.
18. Tandem Intramolecular Silylformylation-Allylsilylation Zacuto, M. J.; Leighton, J. L. J. Am. Chem. Soc. 2000, 122, 8587-8588.
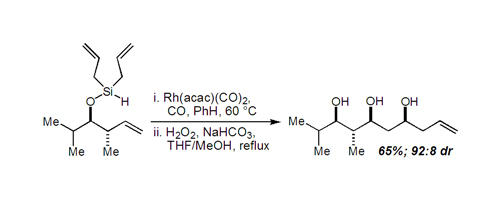
17. Stereoconvergent Palladium-Catalyzed Carbonylation of both E and Z Isomers of a 2-Trifloxy-1,3-Butadiene Bio, M. M.; Leighton, J. L. Org. Lett. 2000, 2, 2905-2907.
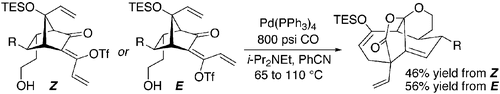
16. Oxymercuration of Homoallylic Alcohol-Derived Hemiacetals: Diastereoselective Synthesis of Protected 1,3-Diols Sarraf, S. T.; Leighton, J. L. Org. Lett. 2000, 2, 403-405.
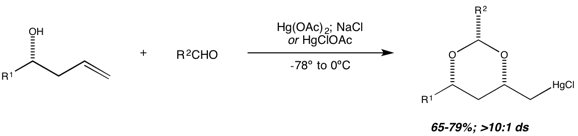
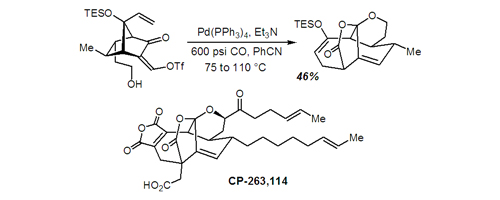
15. An Approach to the Synthesis of CP-263,114: A Remarkably Facile Silyloxy-Cope Rearrangement Bio, M. M.; Leighton, J. L. J. Am. Chem. Soc. 1999, 121, 890-891.
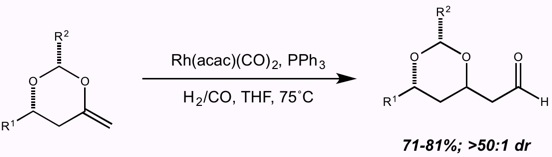
14. Unusual Conformational Effects on the Regioselectivity of Olefin Insertion in the Rhodium-Catalyzed Hydroformylation of 4-Methylene-1,3-Dioxanes Sarraf, S. T.; Leighton, J. L. Tetrahedron Lett. 1998, 39, 6423-6426.
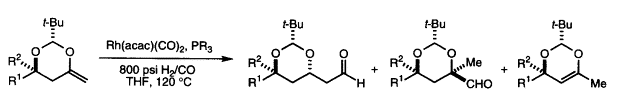
13. Rhodium-Catalyzed Intramolecular Silylformylation of Alkenes Leighton, J. L.; Chapman, E. J. Am. Chem. Soc. 1997, 119, 12416-12417.
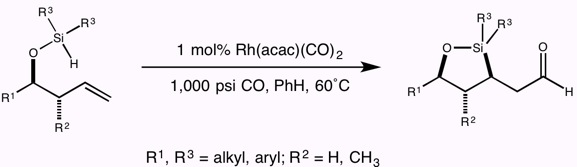
12. Highly Diastereoselective Rhodium-Catalyzed Hydroformylation of Enol Ethers: A Carbonylation-Based Approach to Catalytic Aldol Synthesis Leighton, J. L.; O'Neil, D. N. J. Am. Chem. Soc. 1997, 119, 11118-11119.
Jim's Graduate and Post-Doctoral Work
11. On the Mechanism of Asymmetric Nucleophilic Ring-Opening of Epoxides Catalyzed by (Salen)Cr(III) Complexes Hansen, K. B.; Leighton, J. L.; Jacobsen, E. N. J. Am. Chem. Soc. 1996, 118, 10924-10925.
10. Efficient Synthesis of (R)-4-((Trimethylsilyl)oxy)-2-cyclopentenone by Enantioselective Catalytic Epoxide Ring Opening Leighton, J. L.; Jacobsen, E. N. J. Org. Chem. 1996, 61, 389-390.
9. Highly Enantioselective Ring Opening of Epoxides Catalyzed by (salen)Cr(III)Complexes Martínez, L. E.; Leighton, J. L.; Carsten, D. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5897-5898.
8. Asymmetric Synthesis of the Squalene Synthase Inhibitor Zaragozic Acid C Evans, D. A.; Barrow, J. C.; Leighton, J. L.; Robichaud, A. J.; Sefkow, M. J. Am. Chem. Soc. 1994, 116, 12111-12112.
7. Development of 1,4-Benzodiazepine Cholecystokinin Type B Antagonists Bock, M. G.; DiPardo, R. M.; Evans, B. E.; Rittle, K. E.; Whitter, W. L.; Garsky, V. M.; Gilbert, K. F.; Leighton, J. L.; Carson, K. L.; Mellin, E. C.; Veber, D. F.; Chang, R. S. L.; Lotti, V. J.; Freedman, S. B.; Smith, A. J.; Patel, S.; Anderson, P. S.; Freidinger, R. M. J. Med. Chem. 1993, 36, 4276-4292.
6. Nanomolar Affinity, Nonpeptide Oxytocin Receptor Antagonists Evans, B. A.; Lundell, G. F.; Gilbert, K. F.; Bock, M. G.; Rittle, K. E.; Carroll, L. A.; Williams, P. D.; Pawluczyk, J. M.; Leighton, J. L.; Young, M. B.; Erb, J. M.; Hobbs, D. W.; Gould, N. P.; DiPardo, R. M.; Hoffman, J. B.; Perlow, D. S.; Whitter, W. L.; Veber, D. F.; Pettibone, D. J.; Clineschmidt, B. V.; Anderson, P. S.; Freidinger, R. M. J. Med. Chem. 1993, 36, 3993-4005.
5. The Asymmetric Synthesis of (+)-Calyculin A, a Nanomolar Phosphatase 1 and 2A Inhibitor Evans, D. A.; Leighton, J. L.; Gage, J. R. in Recent Advances in the Chemistry of Anti-infective Agents, Bentley, P.H. and Ponsford, R. Ed.s, Royal Society of Chemistry, 1993, 117-134.
4. Total synthesis of (+)-calyculin A Evans, D. A.; Gage, J. R.; Leighton, J. L. J. Am. Chem. Soc. 1992, 114, 9434-9453.
3. Asymmetric Synthesis of Calyculin A. 3. Assemblage of the Calyculin Skeleton and the Introduction of a New Phosphate Monoester Synthesis Evans, D. A.; Gage, J. R.; Leighton, J. L. J. Org. Chem. 1992, 57, 1964-1966.
2. Asymmetric Synthesis of Calyculin A. 2. The C26-C37 g-amino acid Fragments Evans, D. A.; Gage, J. R.; Leighton, J. L.; Kim, A. S. J. Org. Chem. 1992, 57, 1961-1963.
1. Orally Active, Nonpeptide Oxytocin Antagonists Evans, B. E.; Leighton, J. L.; Rittle, K. E.; Gilbert, K. F.; Lundell, G. F.; Gould, N. P.; Hobbs, D. W.; DiPardo, R. M.; Veber, D. F.; Pettibone, D. J.; Clineschmidt, B. V.; Anderson, P. S.; Freidinger, R. M. J. Med. Chem. 1992, 35, 3919-3927.
