Metallaboratranes
Metallaboratrane compounds are a class of molecules with
a cage-like structure that feature metal-to-ligand M![]() B s–dative bonds, as illustrated below.
B s–dative bonds, as illustrated below.
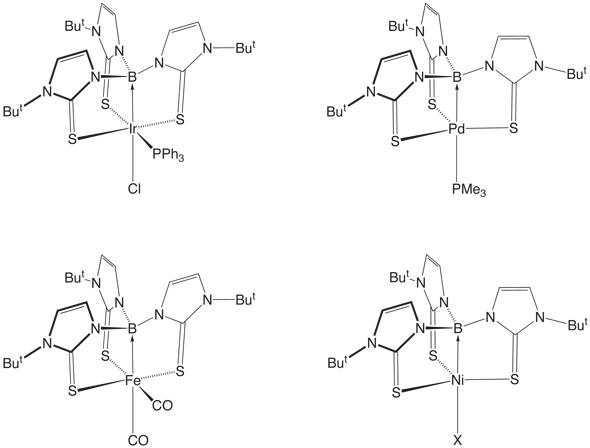
While dative bonding is a common feature of transition metals, the metal is normally the electron pair acceptor, rather than the donor. Of particular interest, therefore, is the nature of the bonding in {[B(mimR)3]M} complexes and the electronic impact that the borane ligand exerts on a metal center. In this regard, two important factors pertaining to the coordination of any ligand to a metal center are the effects that it has on (i) the electron count and (ii) the dn configuration, both of which play an important role in evaluating the stability and reactivity of a molecule.
The essential feature of a general M–B bonding interaction is illustrated by the simplified molecular orbital diagram shown below.
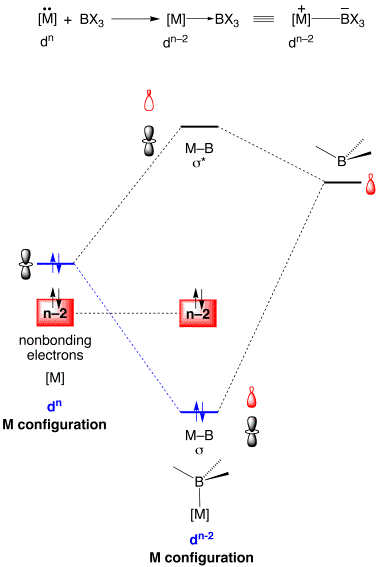
Thus, the interaction between a filled metal based orbital and the empty orbital on boron results in a filled M–B bonding orbital and an unoccupied M–B antibonding orbital. Since the M–B bonding orbital is occupied by a pair of electrons that were originally on the metal, a metal center that originally possessed a dn configuration becomes dn–2 upon coordination to boron. The transition from a dn to dn–2 configuration upon coordination of a BR3 fragment bears a close analogy to the change resulting from the interaction of a metal center with another Lewis acid, namely H+. Thus, it is widely recognized that protonation of a dn metal center results in the formation of a metal–hydride in which the metal center has a dn–2 configuration.
The reactivity of the M![]() B bond in
metallaboratranes has received very little attention. Therefore, we have started to
investigate the chemistry of metallaboratranes, with the intention of
uncovering new reactivity that is associated with cleavage of the M
B bond in
metallaboratranes has received very little attention. Therefore, we have started to
investigate the chemistry of metallaboratranes, with the intention of
uncovering new reactivity that is associated with cleavage of the M![]() B bond.
B bond.
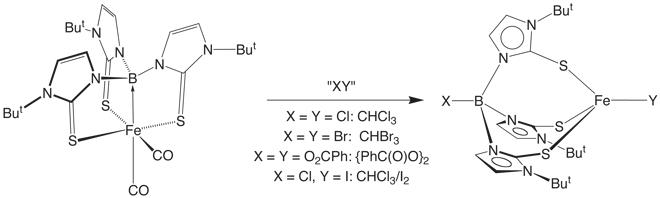
We recently described that the Fe![]() B bond of
the ferraboratrane [k4-B(mimBut)3]Fe(CO)2
could be cleaved by a variety of reagents to give B–functionalized tris(mercaptoimidazolyl)borate complexes of the type [YTmBut]FeZ. For example, [k4-B(mimBut)3]Fe(CO)2
reacts with (i) CHX3 (X = Cl, Br) to give [XTmBut]FeX, and (ii) I2 in CHCl3
to give [ClTmBut]FeI.
B bond of
the ferraboratrane [k4-B(mimBut)3]Fe(CO)2
could be cleaved by a variety of reagents to give B–functionalized tris(mercaptoimidazolyl)borate complexes of the type [YTmBut]FeZ. For example, [k4-B(mimBut)3]Fe(CO)2
reacts with (i) CHX3 (X = Cl, Br) to give [XTmBut]FeX, and (ii) I2 in CHCl3
to give [ClTmBut]FeI.
In a similar manner, the Ni![]() B bond of
the nickel boratrane compounds [k4-B(mimBut)3]NiX (X = Cl, OAc, SCN,
N3) may also be cleaved by suitable reagents to afford B–functionalized derivatives, [YTmBut]NiZ.
B bond of
the nickel boratrane compounds [k4-B(mimBut)3]NiX (X = Cl, OAc, SCN,
N3) may also be cleaved by suitable reagents to afford B–functionalized derivatives, [YTmBut]NiZ.
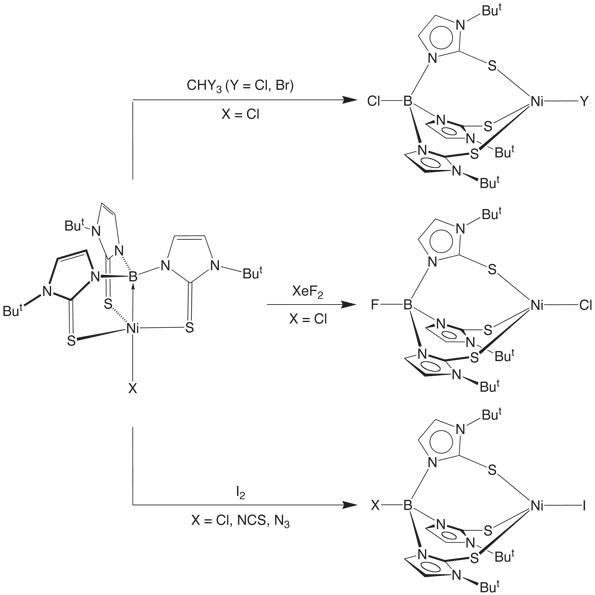
For example, [k4-B(mimBut)3]NiCl reacts with CHCl3, CHBr3, and I2 to give [ClTmBut]NiCl, [ClTmBut]NiBr, and [ClTmBut]NiI, respectively. Likewise, the azide and isothiocyanate complexes [k4-B(mimBut)3]NiN3 and [k4-B(mimBut)3]NiNCS react with I2 to give [N3TmBut]NiI and [SCNTmBut]NiI. In each of these examples, the boron is functionalized by the ligand originally attached to nickel. It is, therefore, significant that [k4-B(mimBut)3]NiCl reacts with XeF2 to yield [FTmBut]NiCl in which the boron is functionalized by the reagent and the chloride ligand remains attached to nickel.
Selected References
“Synthesis and Structural
Characterization of [h3–B,S,S–B(mimR)3]Ir(CO)(PPh3)H
(R = But, Ph) and [h4–B(mimBut)3]M(PPh3)Cl
(M = Rh, Ir): Analysis of The
Bonding in Metal Borane Compounds.”
Victoria K. Landry, Jonathan G. Melnick, Daniela Buccella, Keliang Pang,
Joseph C. Ulichny, and Gerard Parkin Inorg. Chem. 2006, 45,
2588-2597.
“Reactivity of the Metal®BX3
Dative s-Bond: 1,2-Addition Reactions of the Fe®BX3
Moiety of the Ferraboratrane Complex [k4-B(mimBut)3]Fe(CO)2.” Joshua S. Figueroa, Jonathan G. Melnick
and Gerard Parkin Inorg. Chem. 2006,
45, 7056-7058.
“Palladium Complexes with Pd![]() B Dative
Bonds: Analysis of the Bonding in
the Palladaboratrane Compound [k4–B(mimBut)3]Pd(PMe3).” Keliang Pang, Stephanie M. Quan and
Gerard Parkin Chem. Commun. 2006,
5015-5017.
B Dative
Bonds: Analysis of the Bonding in
the Palladaboratrane Compound [k4–B(mimBut)3]Pd(PMe3).” Keliang Pang, Stephanie M. Quan and
Gerard Parkin Chem. Commun. 2006,
5015-5017.
“Reactivity of the Ni![]() B Dative s–Bond in the Nickel Boratrane
Compounds [k4–B(mimBut)3]NiX (X = Cl, OAc, NCS,
N3): Synthesis of a
Series of B-Functionalized Tris(2-mercapto-1-t-butylimidazolyl)borato Complexes,
[YTmBut]NiZ.” Keliang Pang, Joseph M. Tanski, and
Gerard Parkin Chem. Commun. 2008, 1008-1010.
B Dative s–Bond in the Nickel Boratrane
Compounds [k4–B(mimBut)3]NiX (X = Cl, OAc, NCS,
N3): Synthesis of a
Series of B-Functionalized Tris(2-mercapto-1-t-butylimidazolyl)borato Complexes,
[YTmBut]NiZ.” Keliang Pang, Joseph M. Tanski, and
Gerard Parkin Chem. Commun. 2008, 1008-1010.
|
|||
