Bioorganometallic
Chemistry of Mercury
Introduction
Mercury alkyls are potent
neurotoxins. For example, methyl
mercury compounds are responsible for Minamata disease, which caused the death
of almost two thousand people around Minamata Bay (Japan) in the late 1950s
when the residents consumed fish that was contaminated with methyl mercury
compounds released by the Chisso Corporation from 1932 – 1968 (Clarkson,
T. W.; Magos, L. Crit. Rev. Toxicol. 2006, 36, 609).
However, while the initial
outbreak of the Minamata disease was a result of toxic release from a nearby
chemical plant, methyl mercury compounds are naturally occurring! Specifically, [CH3Hg]+ is also introduced into the environment by biomethylation of Hg(II) with
methylcobalamin (i.e. methyl B12)
in sulfate reducing bacteria that live in anoxic aquatic environments (e.g. lake-bottom sediments).
A critical step of mercury
detoxification involves protolytic cleavage of the otherwise inert Hg–R
bond. This is achieved in bacteria
by organomercurial lyase (MerB) which has three cysteine residues at the
active site.
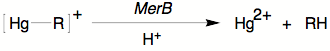
A Functional
Model for MerB
Significantly, we have
obtained a functional model for MerB, in which the Hg–C bond of [TmBut]HgMe is cleaved rapidly by PhSH.
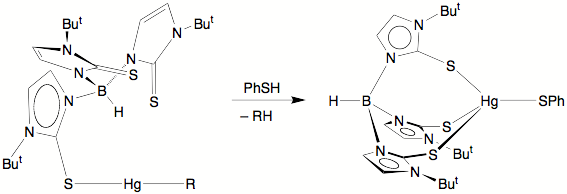
The facility with which the
Hg–C bonds are cleaved under mild conditions is proposed to be a
consequence of the mercury center of two-coordinate [k1–TmBut]HgR being able to access higher
coordination numbers due to the multidentate nature of the [TmBut] ligand.
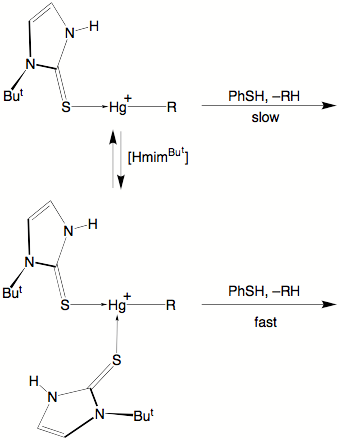
Evidence that increased
coordination promotes the Hg–C protolytic cleavage is provided by the
observation that whereas the reaction of {[HmimBut]HgR}+ with PhSH eliminates RH at elevated
temperatures, the protolytic cleavage occurs at room temperature in the
presence of HmimBut.
Molecular Structure and
Reactivity of Thimerosal (Merthiolate)
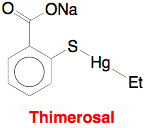
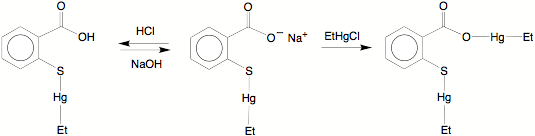
Thimerosal, i.e. sodium ethylmercury thiosalicylate, [(ArCO2)SHgEt]Na, is a pharmaceutical
ingredient that was introduced in the 1930s under the trade name Merthiolate,
and subsequently found applications in a variety of products such as: vaccine
preservatives; antiseptics; contact lens cleaners; soap-free cleansers;
cosmetics; eye, nose and ear drops; and skin test antigens.
In view of the many
applications, and the controversy surrounding its use as a vaccine
preservative, it is rather surprising that there are very few reports
pertaining to the chemistry of thimerosal. Therefore, we have started to investigate the chemistry of
this molecule, including its structural determination by X–ray
diffraction and its analysis by NMR spectroscopy.
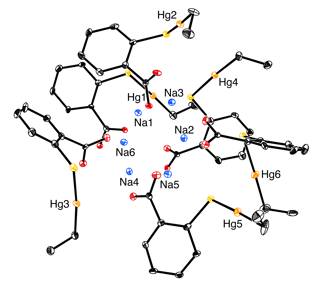
Asymmetric unit of thimerosal.
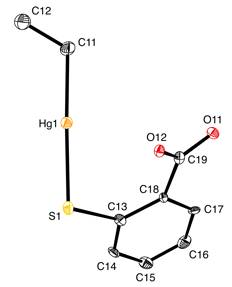
Molecular structure of one of the anions of
thimerosal in the asymmetric unit.
With respect to reactivity,
the carboxylate oxygen of thimerosal, [(ArCO2)SHgEt]Na, is subject to facile electrophilic attack by H+ and [HgEt]+ to give (ArCO2H)SHgEt
and [(ArCO2HgEt)SHgEt]2,
respectively. X–ray
diffraction demonstrates that (ArCO2H)SHgEt
exists as a hydrogen bonded dimer in the solid state whereas [(ArCO2HgEt)SHgEt]2 is
tetranuclear, with the mercury centers being connected by bridging carboxylate
groups.
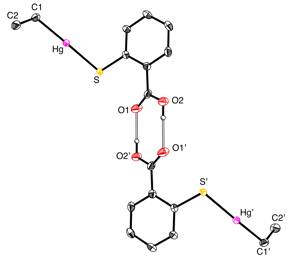
Molecular structure of (ArCO2H)SHgEt.
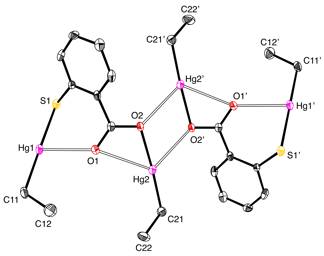
Molecular structure of [(ArCO2HgEt)SHgEt]2.
On the Chalcogenophilicity of
Mercury: Evidence for a Strong Hg–Se
Bond in [TmBut]HgSePh and
its Relevance to the Toxicity of Mercury
While the potent toxicity
of mercury is often associated with its high affinity for sulfur, such that it
binds effectively to the cysteine residues in proteins and enzymes (note that the
term “mercaptan” is an abbreviated form of “mercurium captans”, which is Latin
for “seizing mercury”), another mechanism has been attributed to its impact on
the biochemical roles of selenium. Specifically, the toxicity of mercury has also been attributed to (i) the interaction between between Hg(II) and selenium
compounds reducing the bioavailability of selenium via the formation of insoluble mercury selenide species
and (ii) mercury binding to the
active sites of selenoenzymes, thereby inhibiting their functions.
The preference of mercury to bind selenium over
sulfur has been addressed by examining a series of chalcogenolate complexes
which are supported by the the tris(2-mercapto-1-t-butyl–imidazolyl)hydroborato
ligand, i.e. [TmBut]MEPh
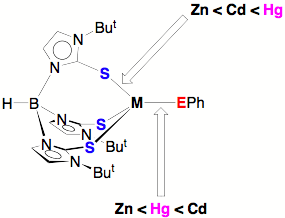
(M = Zn, Cd, Hg; E = S, Se, Te). Interestingly, we observe that the difference in Hg–EPh and
Cd–EPh bond lengths in these complexes is a function of the chalcogen and
that the Hg–SePh and Hg–TePh bonds are much shorter than would be
predicted on the basis of the covalent radii of the chalcogens.
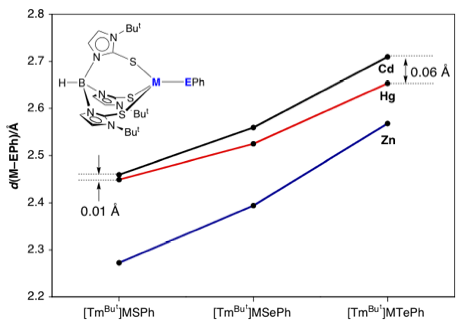
Variation
of M–EPh bond lengths. Note
how the difference between Hg–E and Cd–E bond lengths increases as
the chalcogen becomes heavier.
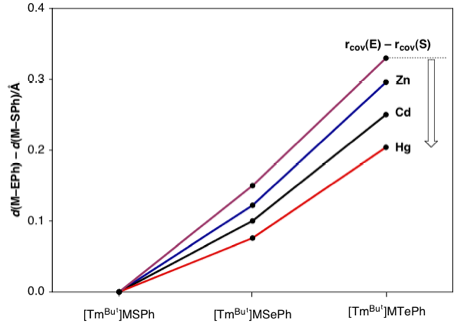
Relative M–EPh bond lengths and the values
predicted on the basis of the covalent radii of S, Se, Te. All
M–SePh and M–TePh bond lengths are shorter than predicted on the
basis of the value for the M–SPh bond length and the change in covalent
radius of the chalcogen, but the Hg–SePh
and Hg–TePh bonds are exceptionally short.
The structural study suggests that while mercury is
often described as being thiophilic, it actually has a greater
selenophilicity, in the sense that the Hg–Se bond is unusually
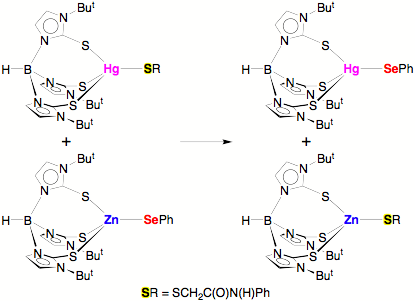
short. Further evidence in support
of this notion was obtained by demonstrating that the selenolate ligand of [TmBut]ZnSePh transfers from zinc to
mercury in [TmBut]HgSR’,
with the thiolate moving to zinc.
Which Element is Bigger: Mercury or Cadmium?
Covalent radii are obtained by analyzing the bond
lengths between different pairs of atoms in a large series of compounds to
obtain a set of self-consistent values (see, for example Pyykkö et al Chem.
Eur. J. 2009, 15, 186-197
and Cordero et al Dalton Trans. 2008,
2832–2838). These radii are
used to provide an estimate of the length of a covalent bond between a pair of
atoms, and while deviations with experimental values may be observed,
differences in closely related compounds are expected to be minimal. However, analysis of a series of [TmBut]MEPh (M = Zn, Cd, Hg; E = S, Se,
Te) compounds reveals an interesting subtlety concerned with the notion of the
covalent radius of an atom, i.e. the apparent covalent radius
of the metal in these complexes is not only molecule dependent, but is also
dependent on the nature of the bond.
Specifically, whereas the Hg–EPh bonds are shorter than the
corresponding Cd–EPh bonds (see above Figure), the Hg–S bonds
involving the [TmBut]
ligand are longer than the corresponding Cd–S bonds (see below
Figure).
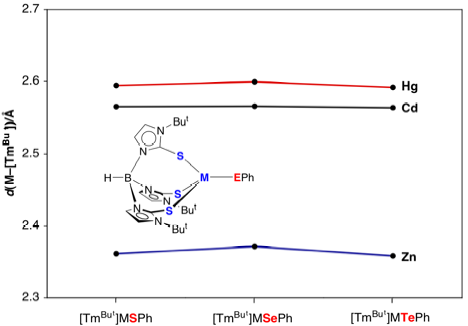
Variation
of M–S bond lengths involving the [TmBut] ligand. Note that all Hg–E bonds are longer than the corresponding
Cd–E bond.
Thus, if one was to ask a simple question “which is bigger, mercury or cadmium”,
one would conclude that mercury is bigger if one considered the M–S bonds
involving the [TmBut]
ligand, whereas one would conclude that cadmium is bigger if one considered the
M–EPh bonds.
Selected
References
“Cleaving
Mercury–Alkyl Bonds: A Functional Model for Mercury Detoxification by MerB.” Jonathan G. Melnick and Gerard Parkin Science 2007, 317, 225-227.
“Molecular Structures of Thimerosal (Merthiolate) and Other Arylthiolate Mercury Alkyl Compounds.” Jonathan G. Melnick, Kevin Yurkerwich, Daniela Buccella, Wesley Sattler and Gerard Parkin, Inorg. Chem. 2008, 47, 6421-6426.
“Molecular Structures of Protonated and Mercurated Derivatives of Thimerosal.” Wesley Sattler, Kevin Yurkerwich and Gerard Parkin Dalton Trans. 2009, 4327-4333.
“Synthesis,
Structure and Reactivity of Two–Coordinate Mercury Alkyl Compounds with
Sulfur Ligands: Relevance to
Mercury Detoxification.” Jonathan G. Melnick, Kevin Yurkerwich and Gerard
Parkin, Inorg. Chem. 2009,
48, 6763-6772.
“On the
Chalcogenophilicity of Mercury: Evidence for a Strong Hg–Se Bond in [TmBut]HgSePh and its Relevance to the
Toxicity of Mercury.” Jonathan G. Melnick, Kevin Yurkerwich and Gerard Parkin J.
Am. Chem. Soc. 2010, 132, 647–655.
|
|||
