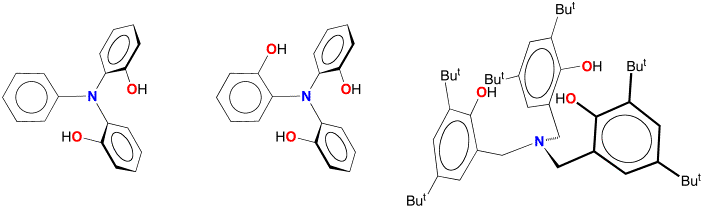
Multidentate Phenols
Alkoxide and aryloxide ligands have been employed
extensively in inorganic chemistry due to their ability to stabilize a variety
of coordination environments.
Specifically, alkoxide and aryloxide ligands are electronically
versatile due to the availability of two lone pairs on the oxygen atom that
allow them to function as one–electron (X), three–electron (LX), or
five–electron (L2X) donors depending upon the electronic needs
of the metal center. We are
interested in developing the application of multidentate aryloxide ligands,
such as those derived from the below phenols, to the chemistry of main group
and transition metals.
Applications of Multidentate Aryloxide Ligands to
Tungsten Chemistry
With respect to bis(aryloxide) ligands, the majority of
studies have been devoted to biphenolate or binaphtholate derivatives in which
the two aryloxide moieties are directly linked together, but more recently
attention has been given to derivatives in which the aryloxide groups are
attached by a linker. For this
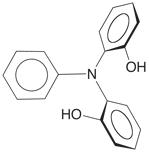
reason, we are interested in the application of the phenylimino-bridged
diphenol PhN(o–C6H4OH)2.
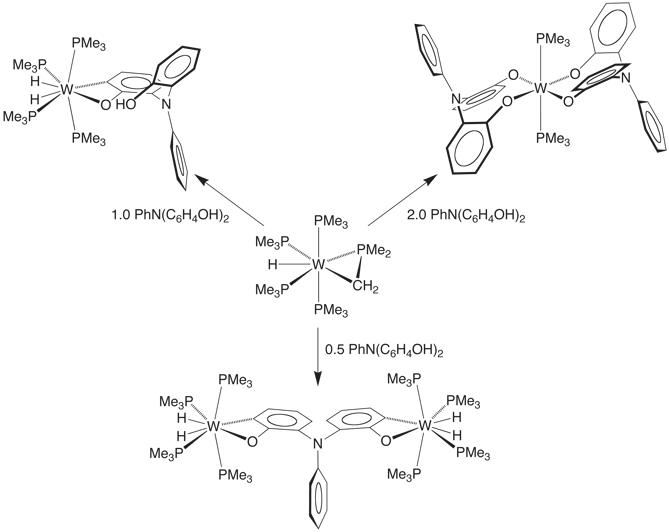
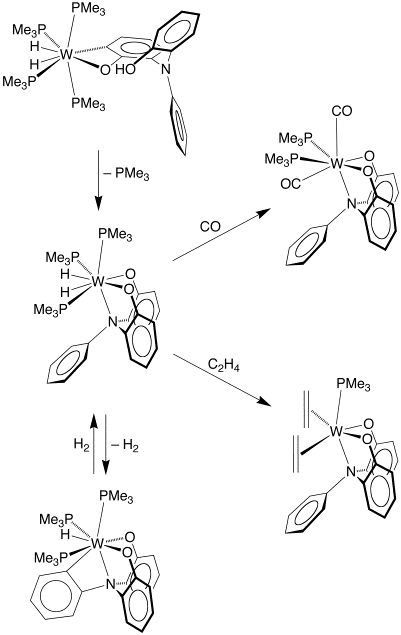
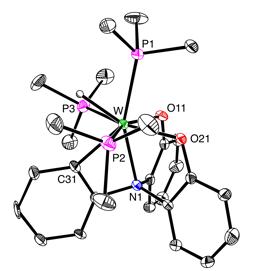
For example, treatment of W(PMe3)4(h2–CH2PMe2)H with PhN(o–C6H4OH)2 results in a series of O–H and C–H bond activation reactions to give products that feature a variety of interesting coordination modes. In particular, the complex [k4–N(C6H4)(C6H4O)2]W(PMe3)3H demonstrates that tetradentate tripodal ligand derived from C–H bond cleavage of the phenyl group.
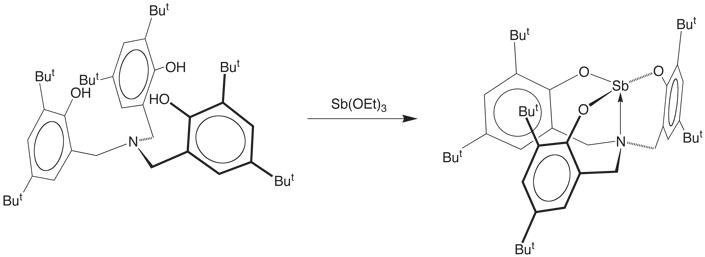
We have also applied multidentate phenoxide ligands shown
above to antimony chemistry. For
example, the antimony aryloxide compound [k4–N(CH2ArBut2O)3]Sb
may be readily obtained via the reaction
of Sb(OEt)3 with tris(3,5-di-t-butyl-2-hydroxybenzyl)amine,
N(CH2ArBut2OH)3.
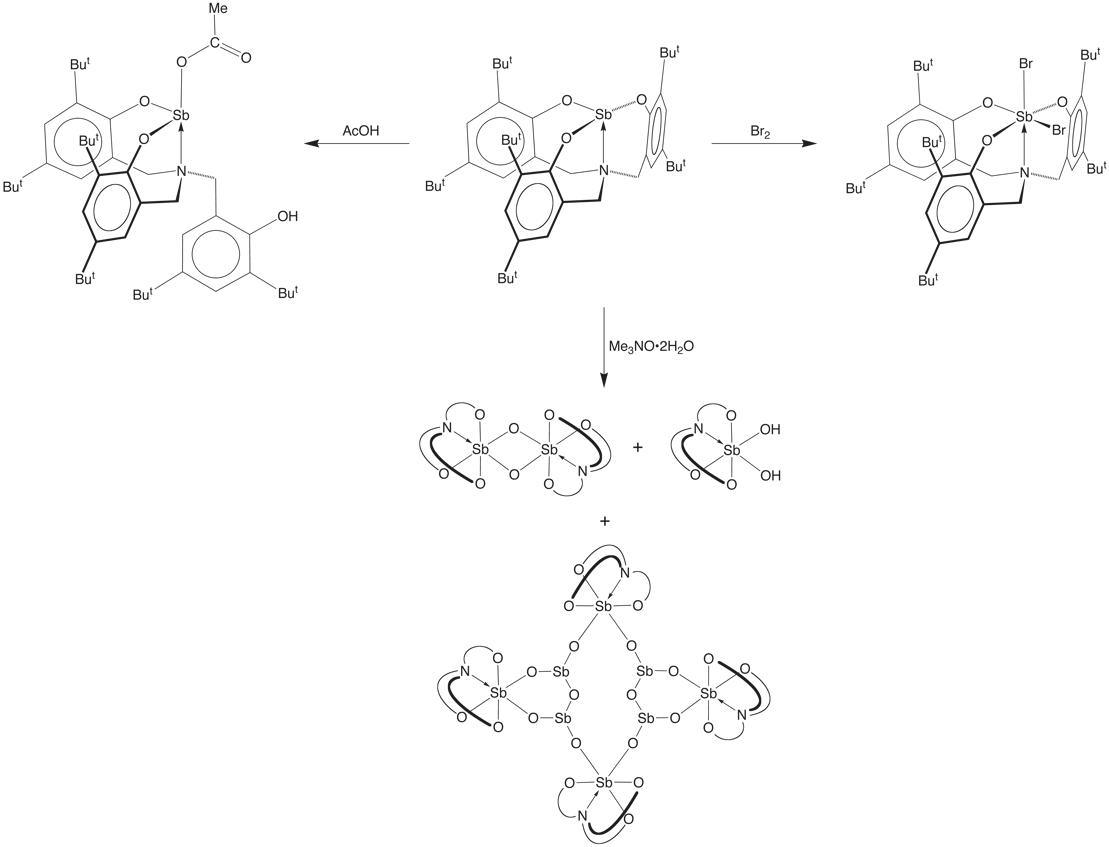
Treatment of [k4–N(CH2ArBut2O)3]Sb
with AcOH cleaves one of the Sb–O bonds to give [k3–N(CH2ArBut2O)2(CH2ArBut2OH)]Sb(k1–O2CMe), while
Br2 undergoes oxidative addition to give N(CH2ArBut2O)3]SbBr2,
and Me3NO•2H2O
yields the oxo and hydroxo complexes {[k4–N(CH2ArBut2O)3]Sb(m–O)}2, [k4–N(CH2ArBut2O)3]Sb(OH)2,
and {[k4–N(CH2ArBut2O)3]SbVO}4{SbIII4O6}.
Selected References
“Synthesis and Structural Characterization of Tris(phenolate)amine Complexes of Antimony Derived from [k4–N(CH2ArBut2O)3]Sb.” Bryte V. Kelly, Edward C. Weintrob, Daniela
Buccella, Joseph M. Tanski, and Gerard Parkin Inorg. Chem. Commun. 2007, 10, 699-704.
“C–H versus O–H Bond Cleavage Reactions of Bis(2–hydroxyphenyl)phenylamine, PhN(o–C6H4OH)2: Synthesis and Structural Characterization of Mononuclear and Dinuclear Tungsten Aryloxide Complexes Which Exhibit Bidentate, Tridentate and Tetradentate Coordination Modes.” Bryte V. Kelly, Joseph M. Tanski, Kevin E. Janak and Gerard Parkin Organometallics 2006, 25, 5839-5842.
“Multidentate Aryloxide and Oxo–Aryloxide Complexes of Antimony: Synthesis and Structural Characterization of [h4–N(o–C6H4O)3]Sb(OSMe2), {{[ h3–N(o–C6H4OH)(o–C6H4O)2]Sb}2(m2–O)}2 and {[h3–PhN(o–C6H4O)2]Sb}4(m3–O)2.” Joseph M. Tanski, Bryte V. Kelly and Gerard Parkin Dalton Trans. 2005, 2442-2447.
“Synthesis and Structural Analysis of Bis(2–hydroxyphenyl)phenylamine, PhN(o–C6H4OH)2: Comparison with Tris(2-hydroxyphenyl)amine N(o–C6H4OH)3.” Bryte V. Kelly, Joseph M. Tanski, Mary Beth Anzovino and Gerard Parkin J. Chem. Crystallogr. 2005, 35, 969-981.
“Antimony Ethylene Glycolate and Catecholate Compounds: The Structural Characterization of Polyesterification Catalysts.” Shannon M. Biros, Brian M. Bridgewater, Adriel Villeges-Estrada, Joseph M. Tanski and Gerard Parkin Inorg. Chem. 2002, 41, 4051-4057.
|
|||
