Bioinorganic Chemistry of Zinc
Introduction
Zinc is essential to all forms of life, with an average adult human containing ca. 3 grams of zinc. The influence of zinc derives from its presence in enzymes, with functions that are both structural and catalytic. An understanding of the multifaceted roles that zinc plays in biological systems requires a correspondingly detailed appreciation of how the chemistry of zinc is modulated by its coordination environment.
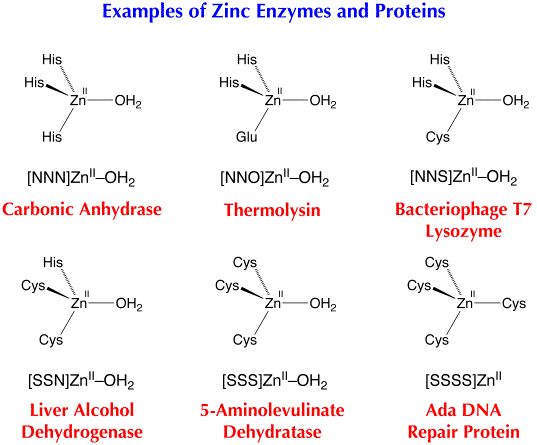
The most common motif structural in zinc enzymes is one in which a tetrahedral zinc center is attached to the protein backbone by three amino acid residues, with the fourth site being occupied by the catalytically important water (or hydroxide) ligand, [{XYZ}ZnII–OHn], as illustrated below.
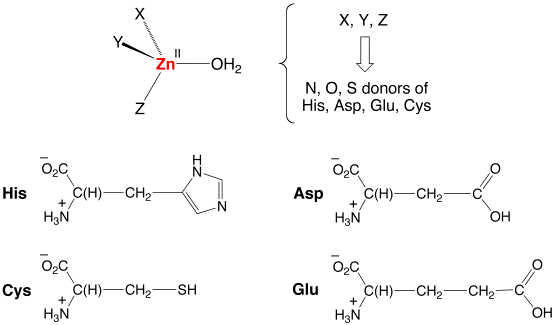
The residues which bind zinc to the protein are typically a combination of His (N), Glu (O), Asp (O), or Cys (S), which provide nitrogen, oxygen, and sulfur donors. Despite the overall similarity of many zinc enzyme active sites in terms of their common tetrahedral [{XYZ}ZnII–OH2] structures, each zinc enzyme performs a different function.
Synthetic Analogues of Zinc Enzymes
Insight into the structures and mechanisms of action of enzymes is often obtained by studying synthetic analogues, i.e. small molecules that resemble the structural and functional sites of the enzymes. To achieve this objective, we have made considerable use of tripodal ligands to provide the requisite X, Y and Z donor groups to mimic the three protein residues that bind zinc at the active site. In particular, tris(pyrazolyl)borate, [TpRR’], and tris(mercaptoimidazolyl)borate, [TmR], ligands respectively provide three nitrogen and three sulfur donors to emulate zinc enzymes and proteins with nitrogen-rich (e.g. carbonic anhydrase) and sulfur-rich active sites (e.g. Ada DNA repair protein).
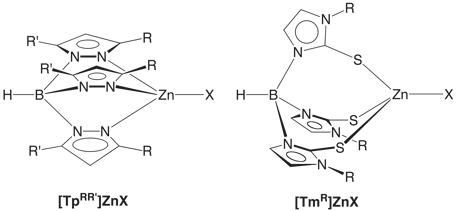
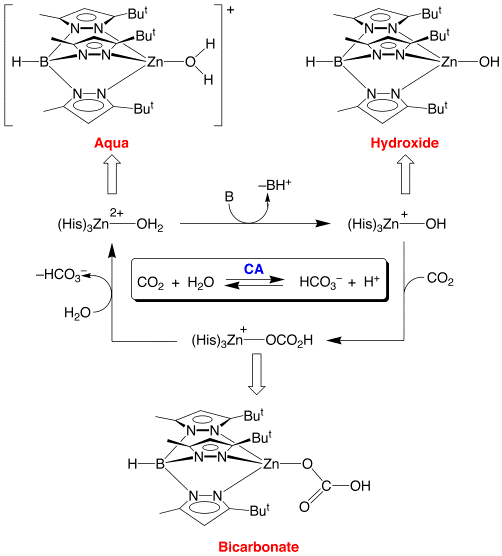
For example, the tris(pyrazolyl)hydroborato system has allowed direct observation of all three zinc species that correspond to the proposed zinc intermediates of the mechanism of action of carbonic anhydrase, namely aqua, hydroxide, and bicarbonate complexes.
Selected References
“Applications of Tripodal [S3] and [Se3]
L2X Donor Ligands to Zinc, Cadmium and Mercury Chemistry:
Organometallic and Bioinorganic Perspectives.” Gerard Parkin New J. Chem. 2007, 31,
1996-2014.
“Synthetic Analogues Relevant to the Structure and
Function of Zinc Enzymes” by Gerard Parkin Chem. Rev. 2004, 104, 699-767.
“Synthetic Analogues of Zinc Enzymes. Gerard Parkin in “Probing of Proteins
by Metal Ions and Their
Low-Molecular-Weight Complexes”, Met. Ions Biol. Syst., Vol. 38, Chapter 14, pp 411-460; A. Sigel and H.
Sigel, eds., M. Dekker, New York, 2001.
“The Bioinorganic Chemistry of Zinc: Synthetic Analogues of Zinc Enzymes
that Feature Tripodal Ligands.” Gerard Parkin Chem. Commun. 2000, 1971-1985.
|
|||
