Metallacarbatranes
Atranes comprise an interesting class of molecules with cage-like structures in which two bridgehead atoms are joined by three three-atom linkers, thereby resulting in a tricyclic motif. If one of the bridgehead atoms is a metal, the transannular interaction can be classified as either an M–L, M–X or M–Z bond.
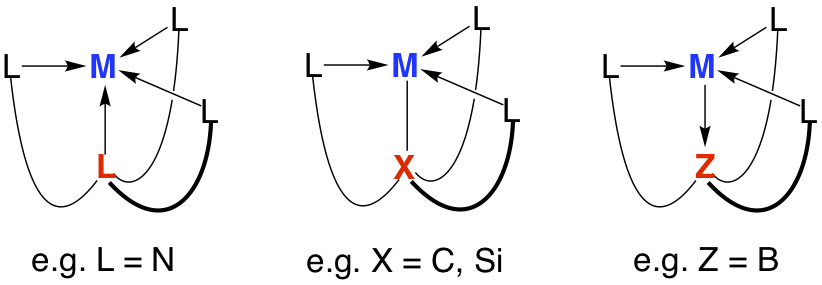
In this regard, the majority of metallatranes possess transannular M¬L or M®Z dative bonds, but we have recently started to explore atranes that feature M–X interactions by employing a series of C3 symmetric tetradentate tripodal ligands. Specific examples include tris(2-pyridylthio)methyl ([Tptm]), tris(1-methylimidazol-2-ylthio)methyl ([TitmMe]), tris(1-isopropylbenzimidazol-2-ylthio)methyl ([TitmPriBenz]), and tris[(1-isopropylbenzimidazol-2-yl)dimethylsilyl]methyl ([TismPriBenz]), which allow for the construction of metallacarbatranes in which the transannular bridge is a M–C bond.
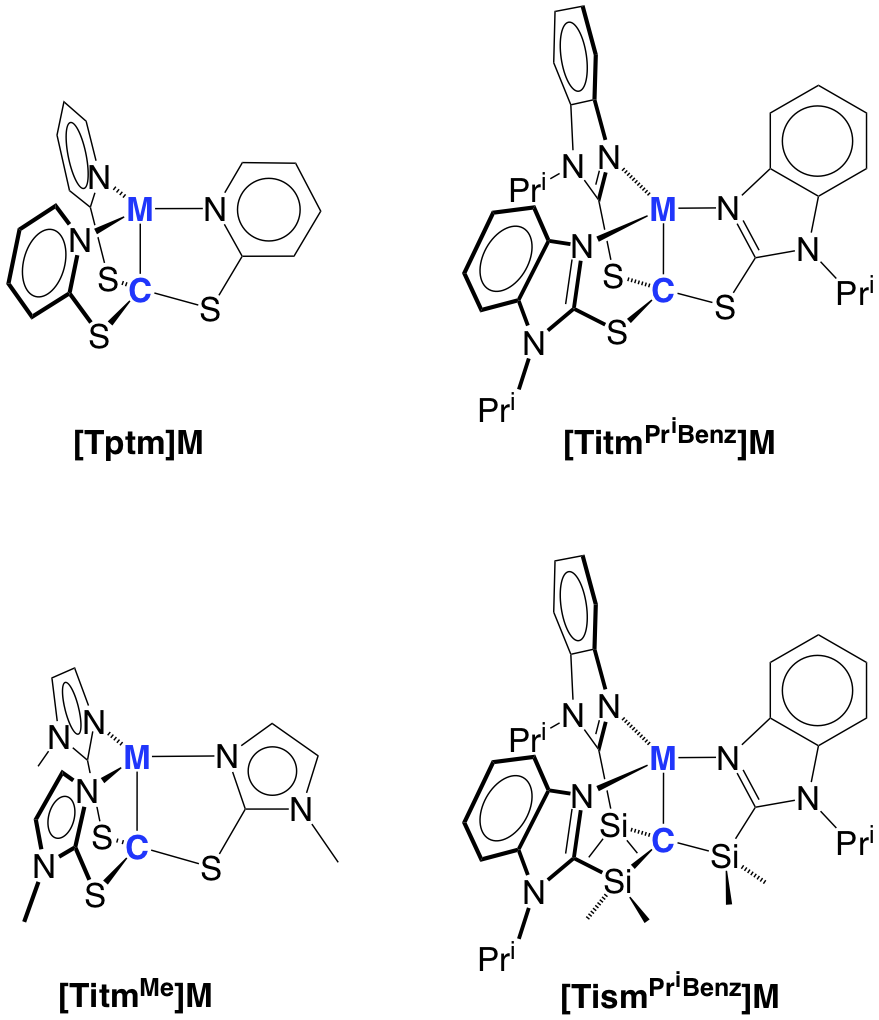
A particularly interesting application of the carbatrane motif is that it has allowed the synthesis of a rare example of a terminal magnesium hydride compound, [TismPriBenz]MgH.
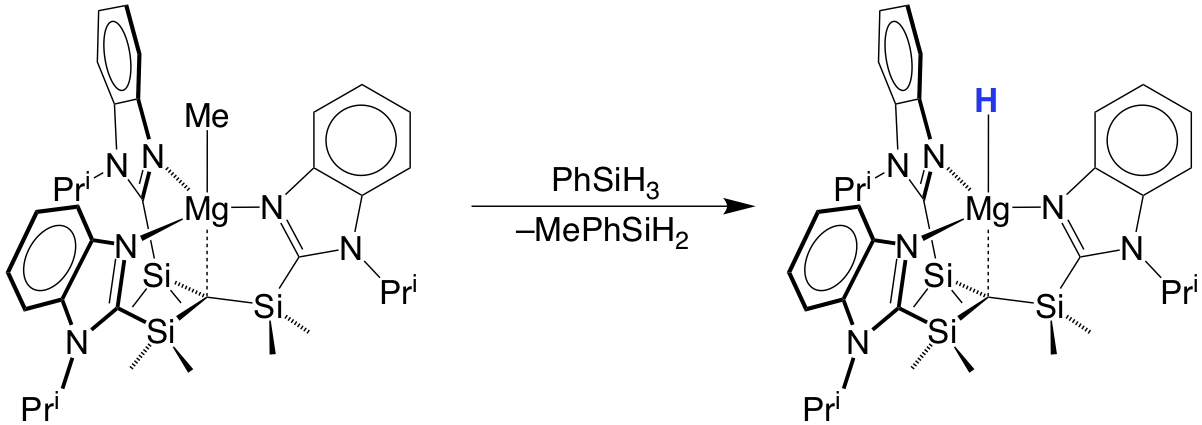
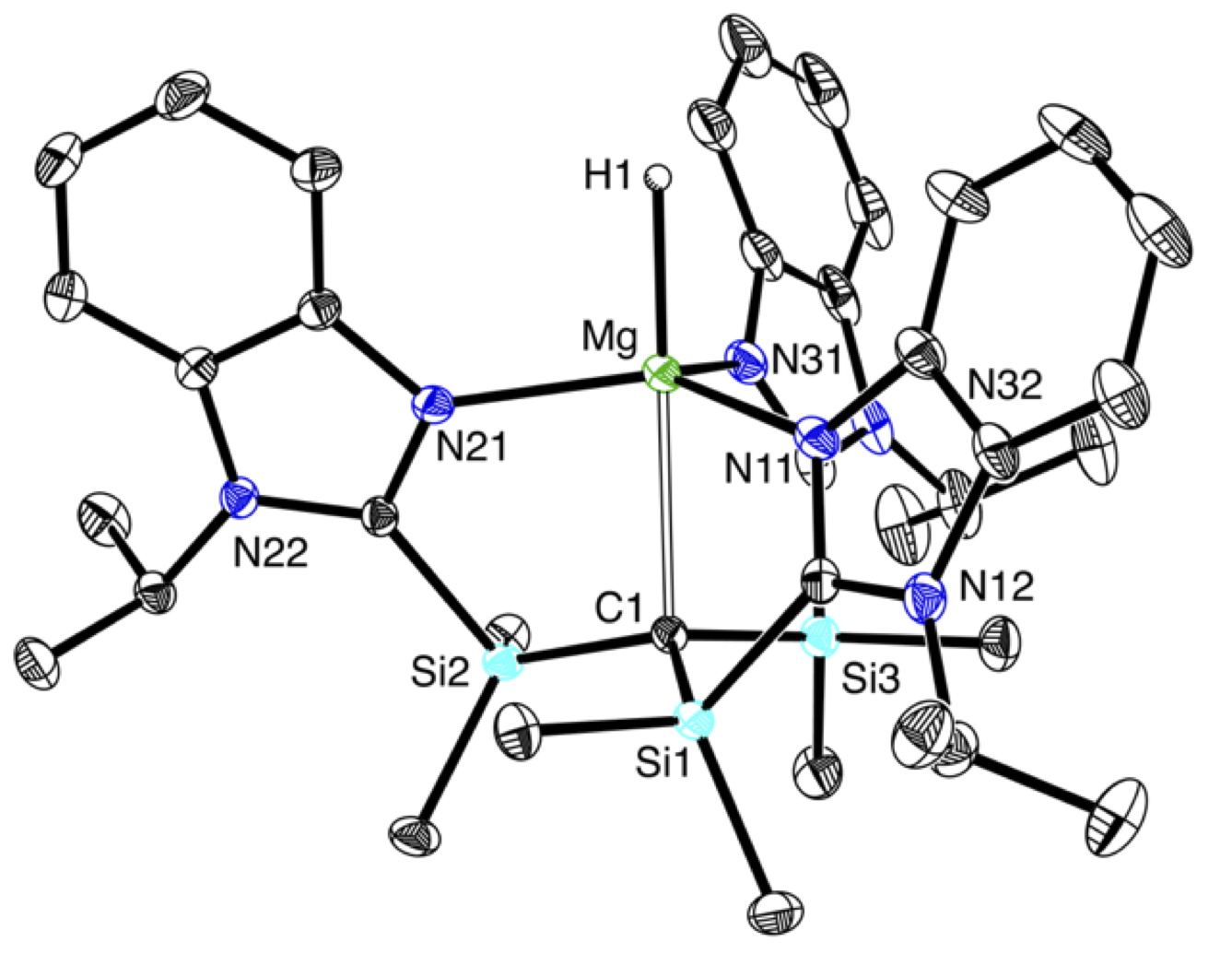
A distinct feature of these magnesium carbatrane compounds is that the Mg–Catrane bonds are much longer than the sum of covalent radii. The long Mg–Catrane distances are consistent with a zwitterionic formalism, i.e. (Mg+ C–). This qualitative view of the bonding is supported by computational studies which demonstrate that the HOMO–1 for [TismPriBenz]MgMe is effectively a lone pair orbital on carbon, with very little contribution from magnesium.
>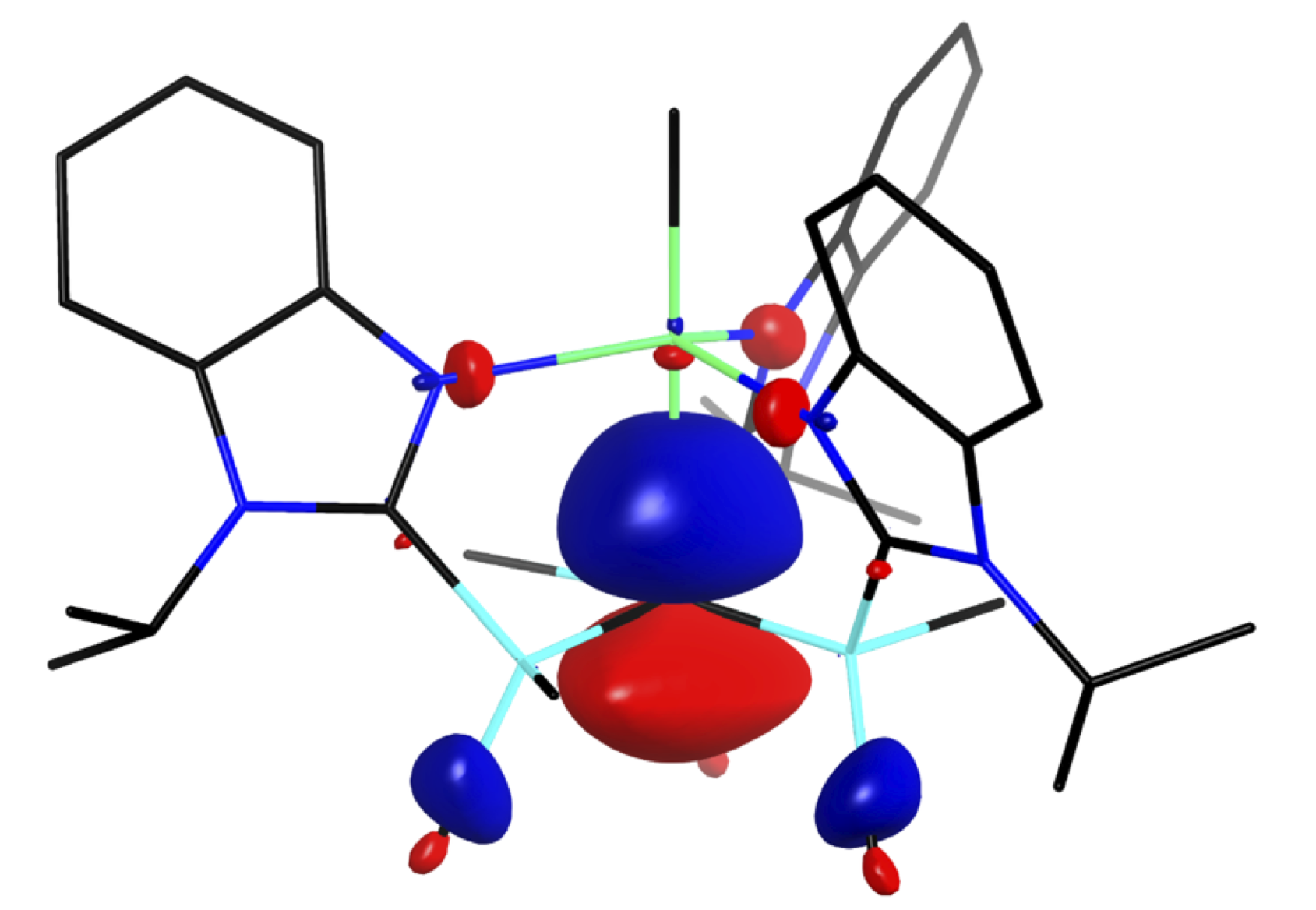
HOMO–1
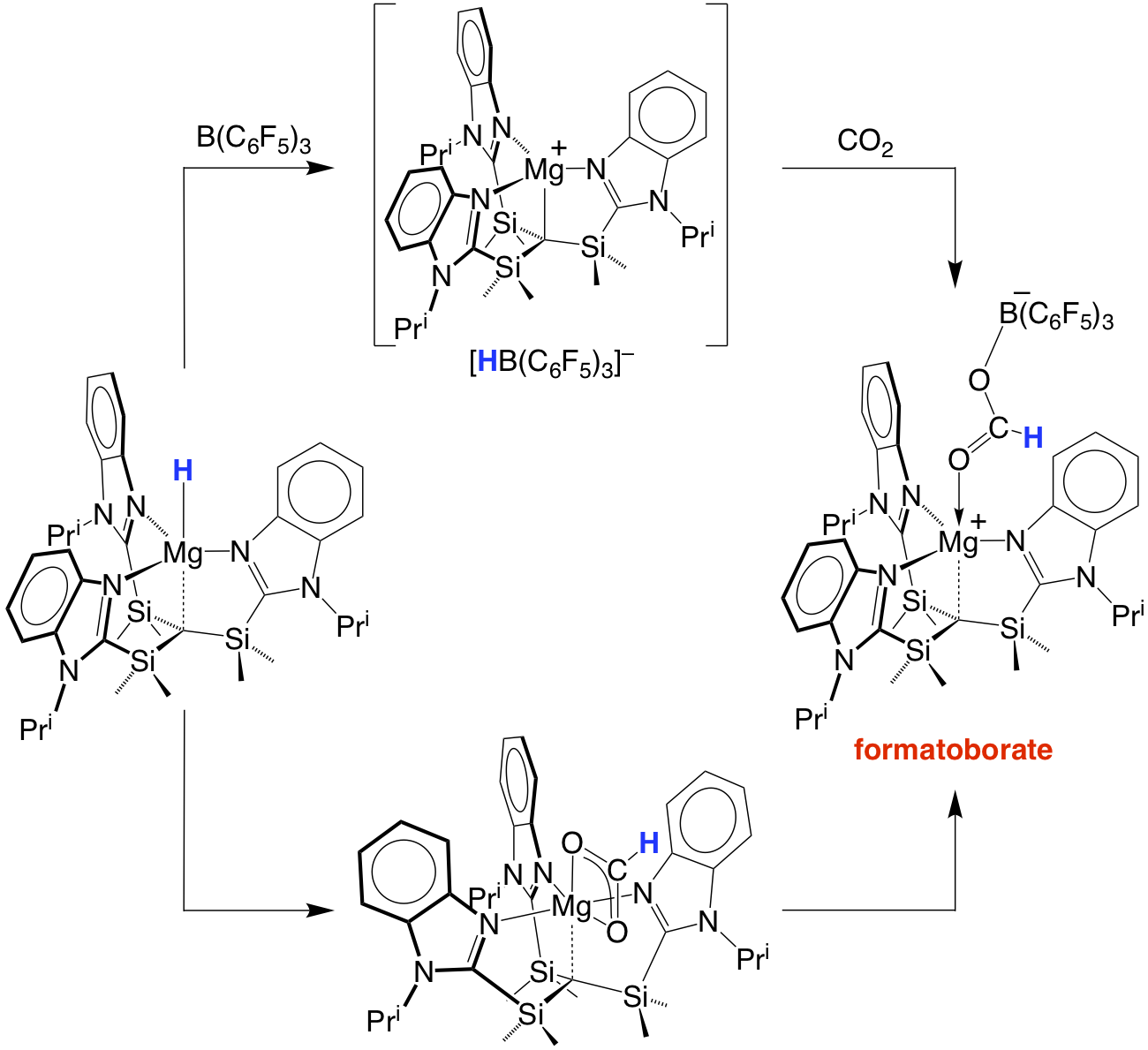
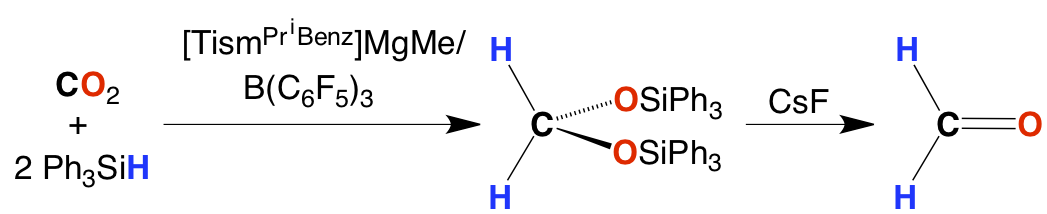
The magnesium hydride complex exhibits novel reactivity. For example, the reactions with CO2 and B(C6F5)3 afford a formatoborate species that provides a means to convert CO2 to formaldehyde.
The copper compound, [TismPriBenz]Cu, also exhibits an interesting bonding feature, namely that the overlap between the carbon 2pz orbital and the copper 3dz2 orbital gives rise to Cu–C bonding and antibonding combinations, of which the latter possesses a significant carbon component rather than copper component.
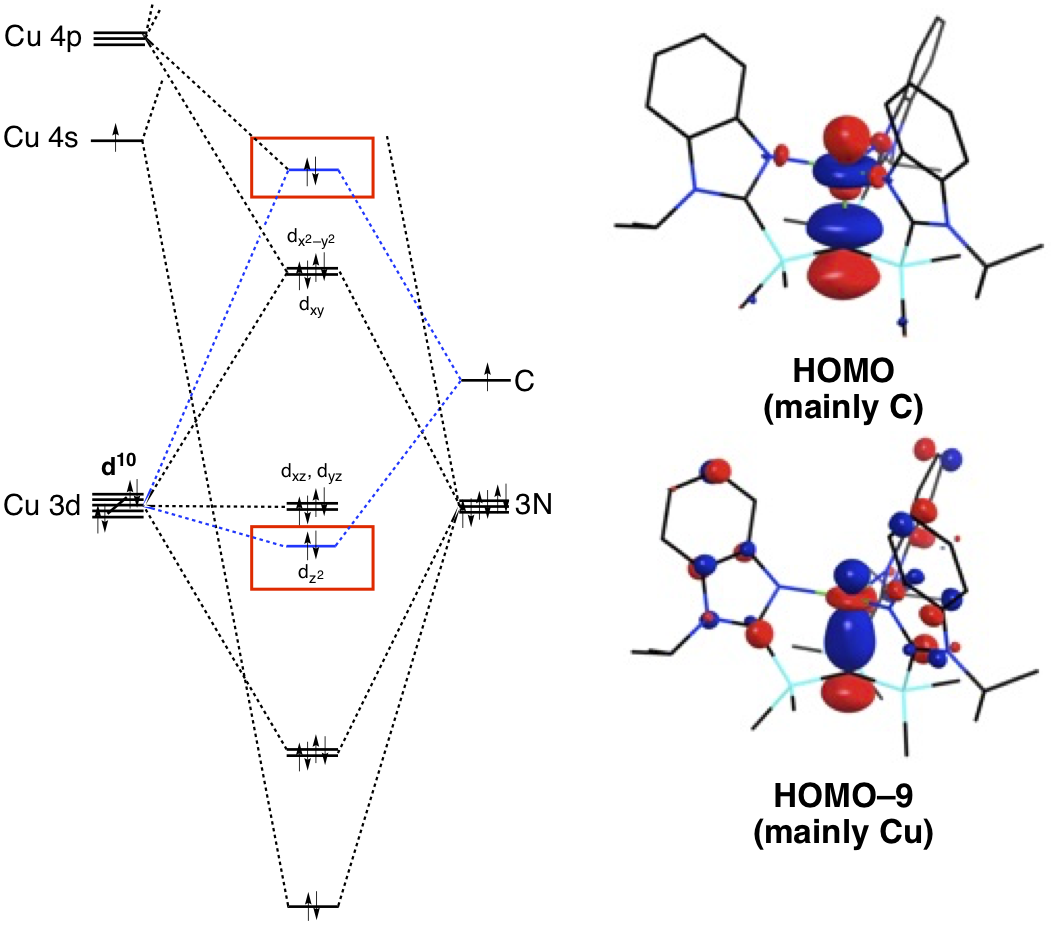
This observation is counter to that observed for most transition metal compounds with s-donor ligands, for which the bonding combination usually possesses more ligand character because the ligand orbitals are typically lower in energy than the metal orbitals. Although situations in which the bonding orbital possesses mainly metal character (and the corresponding antibonding orbital possesses mainly ligand character) are not normally encountered in transition metal chemistry, examples of so-called “inverted ligand fields” have recently been discussed in the literature.
An interesting aspect of the aforementioned tetradentate tripodal ligands is that, in addition to exhibiting a k4-coordination mode that results in an atrane motif, the ligands may also coordinate in a hypodentate k3-manner. For example, the coordination mode of the [Tptm] ligand in [Tptm]ZnX depends on the nature of the X substituent, such that [k4‑Tptm]ZnX (X = F, Cl, Br, I, N3, NCO, OSiR3, O2CH, O2CMe) possess atrane structures, while [k3‑Tptm]ZnX (X = H, Me, N(SiMe3)2) exhibit only k3-coordination of the [Tptm] ligand.
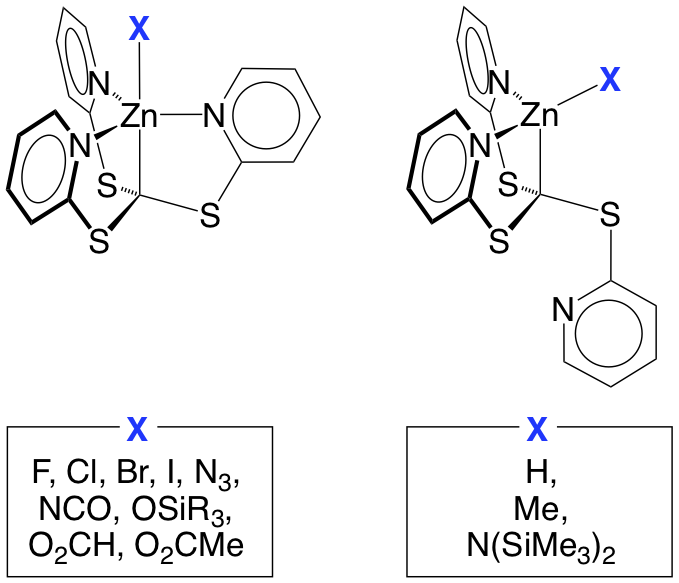
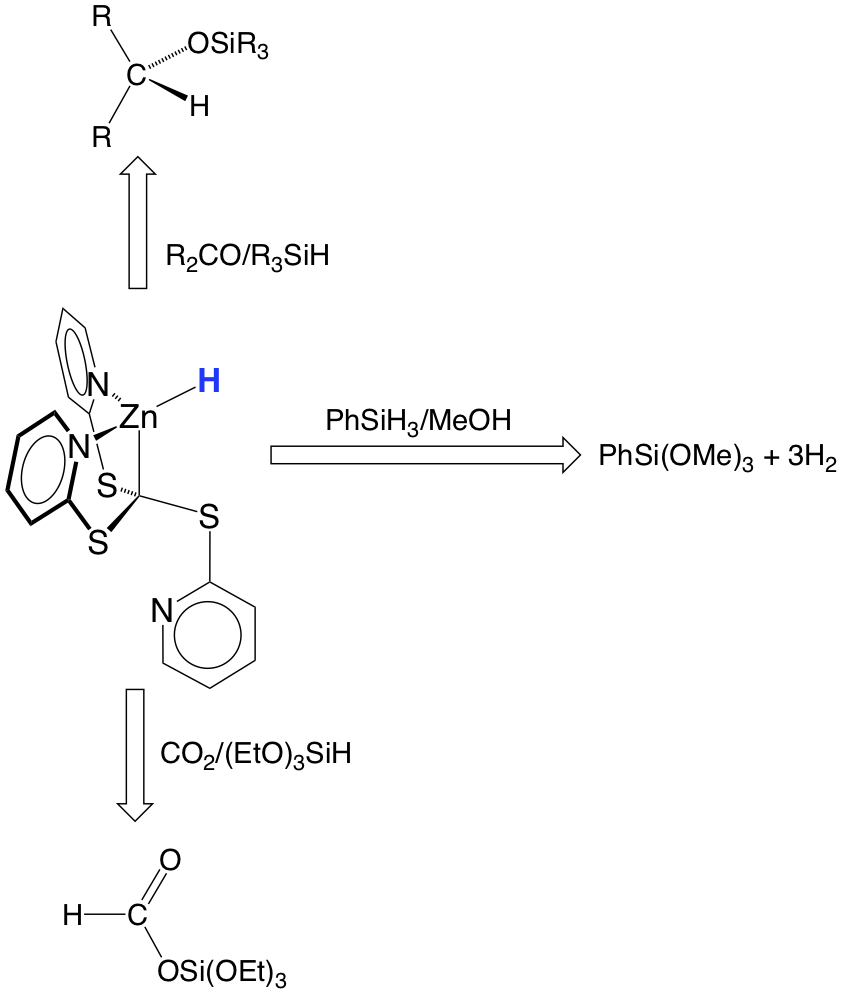
Of these compounds, the zinc hydride derivative, [k3‑Tptm]ZnH, is particularly interesting because it serves as a multifunctional catalyst that is capable of achieving (i) the rapid release of hydrogen by protolytic cleavage of phenylsilane with either water or methanol, and (ii) the hydrosilylation of aldehydes, ketones and carbon dioxide. For example, [k3‑Tptm]ZnH catalyzes the release of three equivalents of H2 from phenylsilane in the presence of methanol with a turnover number of 100,000 and a turnover frequency of 106 h‑1 for the first equivalent. In addition, [k3‑Tptm]ZnH serves as a catalyst for the hydrosilylation of carbon dioxide to give triethoxysilyl formate, which may subsequently be converted into useful chemicals such as ethyl formate or N,N-dimethylformamide.
Selected
References
Ruccolo, S.; Rauch, M.; Parkin, G. "Tris[(1-isopropylbenzimidazol-2-yl)dimethylsilyl]methyl Metal Complexes, [TismPriBenz]M: A New Class of Metallacarbatranes, Isomerization to a Tris(N-heterocyclic carbene) Derivative, and Evidence for an Inverted Ligand Field” Chem. Sci. 2017, 8, 4465-4474.
Rauch, M.; Ruccolo, S.; Parkin, G. “Synthesis, Structure, and Reactivity of a Terminal
Magnesium Hydride Compound with a Carbatrane Motif, [TismPriBenz]MgH:
A Multifunctional Catalyst for Hydrosilylation and Hydroboration” J. Am.
Chem. Soc. 2017, 139, 13264-13267.
Rauch, M.; Parkin, G. “Zinc and Magnesium Catalysts for the Hydrosilylation of Carbon
Dioxide” J. Am. Chem. Soc. 2017, 139, 18162−18165.
Ruccolo, S.; Sattler, W.; Rong, Y.; Parkin, G. “Modulation of Zn–C Bond Lengths
Induced by Ligand Architecture in Zinc Carbatrane Compounds” J. Am. Chem. Soc. 2016, 138,
14542-14545.
Sattler, W.; Parkin, G.
“Synthesis, Structure, and Reactivity of a Mononuclear Organozinc Hydride Complex:
Facile Insertion of CO2 into a Zn–H Bond and CO2-promoted
Displacement of Siloxide Ligands” J. Am. Chem. Soc. 2011, 133, 9708-9711.
Sattler, W.;
Parkin, G. “Zinc Catalysts for On-Demand Hydrogen Generation and Carbon Dioxide
Functionalization” J. Am. Chem.
Soc. 2012, 134, 17462-17465.
Sattler, W.; Ruccolo, S.; Parkin, G. “Synthesis,
Structure, and Reactivity of a Terminal Organozinc Fluoride Compound: Hydrogen Bonding,
Halogen Bonding, and Donor-Acceptor Interactions” J. Am. Chem. Soc.
2013, 135,
18714-18717.
Sattler, W.; Ruccolo, S.; Chaijan, M. R.; Allah, T. N.; Parkin, G. “Hydrosilylation of Aldehydes and Ketones Catalyzed by a Terminal Zinc Hydride
Complex, [k3-Tptm]ZnH” Organometallics
2015, 34, 4717-4731.
Sattler, W.; Parkin, G. “Reduction of Bicarbonate
and Carbonate to Formate in Molecular Zinc Complexes” Catal. Sci. Tech. 2014, 4, 1578–1584.
Sattler, W.; Parkin, G. “Structural
Characterization of Zinc Bicarbonate Compounds Relevant to the Mechanism of Action
of Carbonic Anhydrase” Chem. Sci. 2012,
3, 2015-2019.
Chakrabarti, N.; Sattler, W.; Parkin, G. “Structural Characterization of
Tris(pyrazolyl)hydroborato and Tris(2-pyridylthio)methyl Lithium Compounds:
Lithium in Uncommon Trigonal Pyramidal and Ttrigonal Monopyramidal Coordination
Environments” Polyhedron 2013,
58, 235-246.